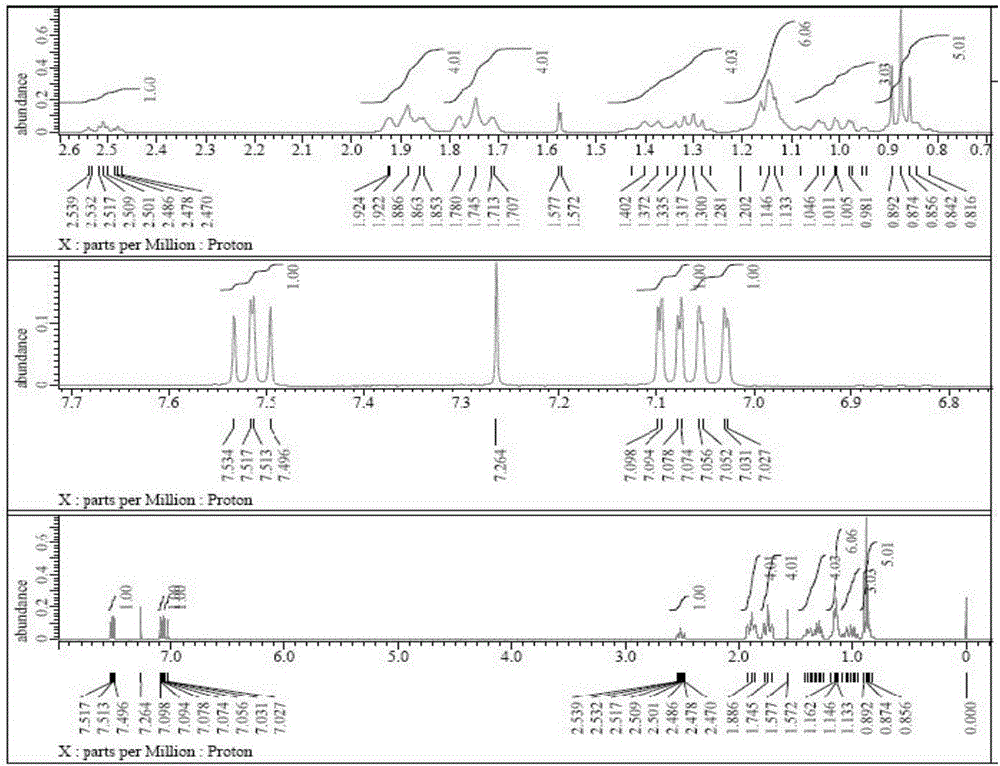
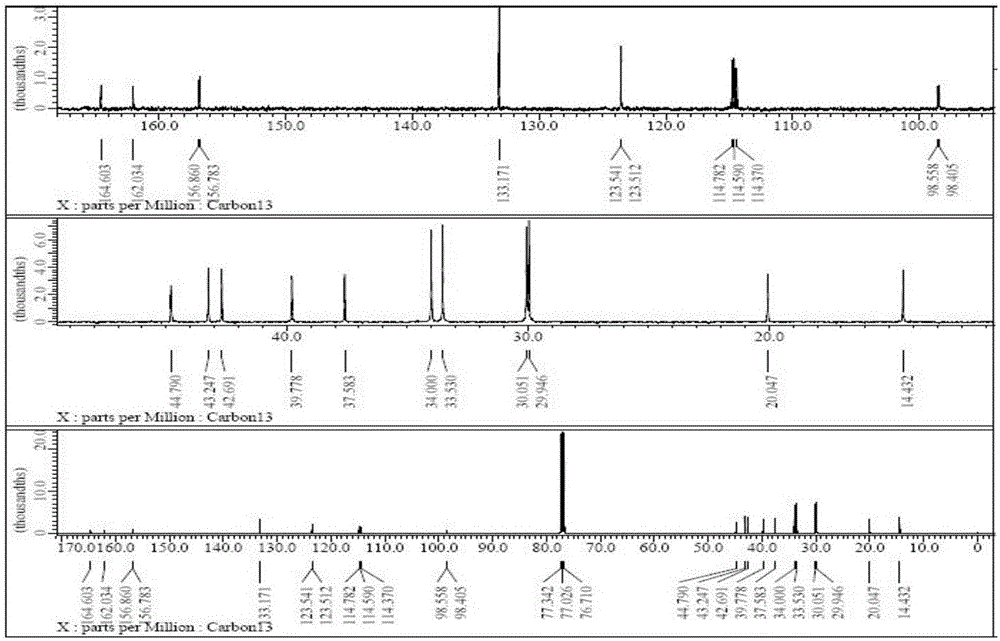
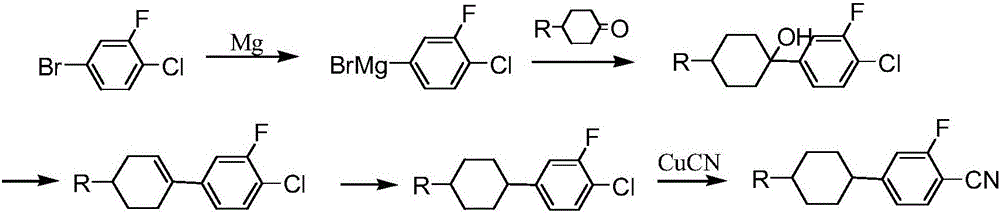
Preparation method of cyclohexyl fluorine-containing cyanophenyl derivative liquid crystalline monomer
A technology of fluorine-containing benzonitrile and liquid crystal monomers, which is applied in the preparation of organic compounds, carboxylic acid nitriles, and liquid crystal materials. It can solve the problems of cumbersome operations, high safety risks, and high production costs, and achieve shortened reaction routes and high activity. High, short response time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] When n=1, R 1 is n-propyl, X is F atom, Y is H atom, and compound IV is 2-fluoro-4-(4'-propyl-bicyclohexyl)-benzonitrile
[0046] (1) In a 2000mL three-necked bottle, add 199g (1.0mol) 2-fluoro-4-bromobenzonitrile, then add 900g tetrahydrofuran, mix well, add 222g (1.0mol) 4-propyl bicyclohexyl ketone, in At a temperature of -60°C to -70°C, add 400mL (1.0mol) of n-butyllithium solution (2.5mol / L) dropwise. 300g of glacial acetic acid with a mass fraction of 20% was hydrolyzed, the hydrolyzed layers were separated, and after the organic phase was washed and neutralized, tetrahydrofuran was removed to obtain 304.2g of light yellow liquid 2-fluoro-4-(4-hydroxyl-4'-propyl-bicyclic Hexyl)-benzonitrile, the yield is 88.7%;
[0047] (2) Add 304.2 g (0.887 mol) of 2-fluoro-4-(4-hydroxyl 4'-propyl-bicyclohexyl)-benzonitrile obtained in step (1) into a 2000 mL three-necked flask, and then add 912.6 g toluene and 30.6g (0.1774mol) p-toluenesulfonic acid, at a temperature of 110...
Embodiment 2
[0050] When n=0, R 1 is n-propyl, X is an F atom, Y is an H atom, and compound IV is 2-fluoro-4-(4'-propyl-cyclohexyl)-benzonitrile
[0051] (1) In a 2000mL three-necked bottle, add 199g (1.0mol) 2-fluoro-4-bromobenzonitrile, then add 900g tetrahydrofuran, mix well, add 140g 4-propyl cyclohexanone (1.0mol), in At a temperature of -60°C to -70°C, add 400mL (1.0mol) of n-butyllithium solution (2.5mol / L) dropwise, and after the dropwise addition is completed and kept warm for 2-3 hours, the metallization reaction is carried out, and the reaction is completed. Pour 300g of glacial acetic acid with a mass fraction of 20% for hydrolysis, hydrolyze and separate layers, wash the organic phase with water to neutralize, and remove tetrahydrofuran to obtain 235.1g of light yellow liquid 2-fluoro-4-(1-hydroxyl-4'-propyl -cyclohexyl)-benzonitrile, the yield is 90.0%;
[0052] (2) Add 235.1g (0.9mol) of 2-fluoro-4-(1-hydroxy-4'-propyl-cyclohexyl)-benzonitrile obtained in step (1) into a 2...
Embodiment 3
[0055] When n=1, R 1 is pentyl, X is F atom, Y is F atom, compound IV is 2,6-difluoro-4-(4'-pentyl-bicyclohexyl)-benzonitrile
[0056] (1) Into a 2000mL three-necked bottle, add 108.5g (0.5mol) 2,6-difluoro-4-bromobenzonitrile, then add 545g tetrahydrofuran, mix well, add 125g (0.5mol) 4-pentylbicyclo For hexyl ketone, add 200mL (0.5mol) of n-butyllithium solution (2.5mol / L) dropwise at a temperature of -60°C to -70°C. , after the reaction is complete, pour 150g of glacial acetic acid with a mass fraction of 20% for hydrolysis, hydrolysis and layering, and after the organic phase is washed with water to neutrality, 174.1g of 2,6-difluoro-4-(4-hydroxy- 4'-pentyl-bicyclohexyl)-benzonitrile, the yield was 89.5%;
[0057] (2) Add 174.1 g (0.45 mol) of 2,6-difluoro-4-(4-hydroxy-4'-pentyl-bicyclohexyl)-benzonitrile obtained in step (1) into a 2000 mL three-necked flask , then add 522.3g toluene and 15.6g (0.09mol) p-toluenesulfonic acid, at 110°C, reflux and separate water to kee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com