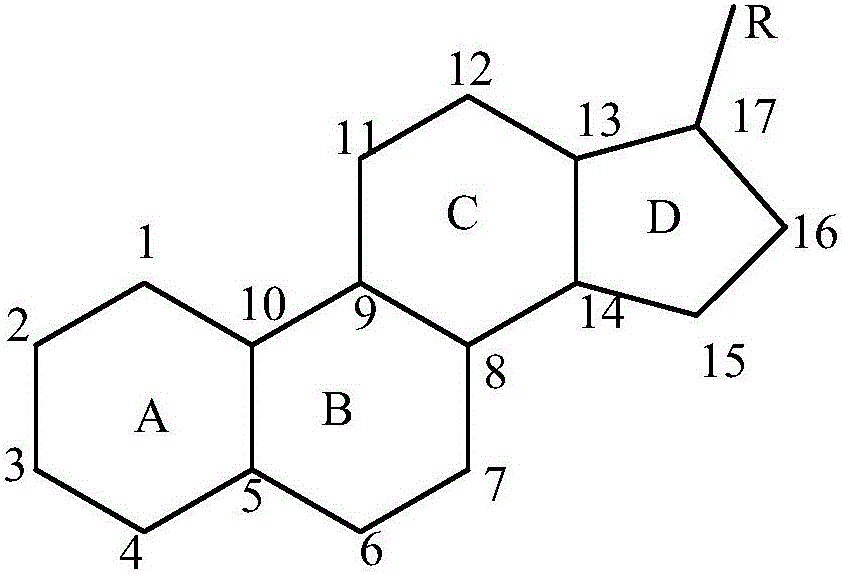
Method for preparing 11Alpha,17Alpha-hydroxyprogesterone
A technology of hydroxyprogesterone and culture medium, which is applied in the field of preparing 11α, 17α-hydroxyprogesterone and steroid intermediates, can solve the problems of bacterial cell damage, inability to recycle, less application of steroid drugs, etc., and achieves low production cost , the effect of saving production costs and high industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Aspergillus ochraceus was cultured on a slant to obtain the strains required for fermentation.
[0027] 1. Preparation of Aspergillus ochraceus slant strain and seed solution
[0028] (1) Incline cultivation
[0029] Incline medium: peeled potato 200g / L, glucose 20g / L, agar 20g / L, natural pH, sterilized at 121℃ for 30min.
[0030] The bacterial species preserved in the glycerol tube is aseptically drawn a certain amount of bacterial liquid and spread evenly on the slant medium, and cultured at 28°C for 4-6 days.
[0031] (2) Seed cultivation
[0032] Seed medium: Glucose 10g / L, corn steep liquor 10g / L, yeast extract 10g / L, natural pH 7.0, sterilized at 121°C for 30min.
[0033] With the strain cultivated in step (1), under aseptic conditions, use an inoculation loop to pick 6 ring spores into 100ml seed medium, inoculate 4 bottles in the same way, and cultivate them at 28°C and 200r / min for 24h , to obtain the seed liquid.
[0034] 2. Fermentation
[0035] Ferment...
Embodiment 2
[0041] The Aspergillus ochraceus bacterial strain is made into seed liquid (the method is the same as in Example 1), and then fermented.
[0042] Fermentation medium: glucose 20g / L, corn steep liquor 20g / L, yeast extract 10g / L, pH value 5.0. The fermenter containing the fermentation medium was sterilized by high-pressure steam at 121° C. for 15 minutes.
[0043]Use a 5L fermenter for biotransformation, with a liquid volume of 3.5L (70%). Transfer the seed liquid into the fermentation medium according to the 10% inoculum volume, at a temperature of 28°C. According to the concentration of the bacteria, the stirring effect and the dissolved oxygen level, Adjust the air ratio and stirring speed, the air ratio control range is 0.7-1.2v / v / min, the speed control range is 200-600r / min. When the incubation time is 18 hours, add dimethylformamide (DMF) 5g / L, substrate 17α-hydroxyprogesterone 20g / L, and take samples at intervals of 12 hours for TLC to detect the conversion. The color d...
Embodiment 3
[0048] The Aspergillus ochraceus bacterial strain is made into seed liquid (the method is the same as in Example 1), and then fermented.
[0049] Fermentation medium: glucose 20g / L, corn steep liquor 20g / L, yeast extract 10g / L, pH value 5.0. The fermenter containing the fermentation medium was sterilized by high-pressure steam at 121° C. for 15 minutes.
[0050] Use a 5L fermenter for biotransformation, with a liquid volume of 3.5L (70%). Transfer the seed liquid into the fermentation medium according to the 10% inoculum volume, at a temperature of 28°C. According to the concentration of the bacteria, the stirring effect and the dissolved oxygen level, Adjust the air ratio and stirring speed, the air ratio control range is 0.7-1.2v / v / min, the speed control range is 200-600r / min. When the incubation time was 18 hours, 5 g / L of nanometer ferric oxide powder with an average particle size of 30 nm and 20 g / L of the substrate 17α-hydroxyprogesterone were added. During this period...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com