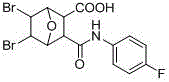
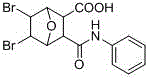
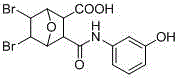
Method for green synthesis of norcantharidin derivative
A green synthesis technology of demethylcantharidin, applied in the direction of organic chemistry, can solve the problems of long reaction time, many reaction steps, high solvent toxicity, etc., and achieve good substrate adaptability, mild reaction conditions, and simple reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022] with 5 mL of CH 2 Cl 2 Add maleic anhydride (10 mmol, 1 g) and freshly distilled furan (20 mmol, 1.4 g) to a 25 mL round bottom flask, stir at room temperature for 24 h, filter, wash with ether, and spin dry. Afterwards, under the protection of nitrogen, the bromine CH 2 Cl 2 The solution was added dropwise to the round-bottomed flask within 1 h. After the dropwise addition, the reaction solution was stirred and reacted at room temperature for 3.5 h. Then filter and filter with CH 2 Cl 2 The solid was washed to obtain a white solid. After the filtrate was spin-dried, it was washed with ether to obtain 7-oxo-bicyclo[2.2.1]heptane-5,6-dibromoic anhydride. Finally, add 7-oxo-bicyclo[2.2.1]heptane-5,6-dibromoic anhydride (1 mmol) and amine compound (0.5 mmol) to the Schlenk containing 2 mL of acetone, and stir at room temperature 1 s, TLC spot plate monitoring, after the reaction, filtered, washed with acetonitrile and dried by infrared. Yield 89%.
[...
Embodiment 2
[0025]
[0026] with 5 mL of CH 2 Cl 2 Add maleic anhydride (10 mmol, 1 g) and freshly distilled furan (20 mmol, 1.4 g) to a 25 mL round bottom flask, stir at room temperature for 24 h, filter, wash with ether, and spin dry. Afterwards, under the protection of nitrogen, the bromine CH 2 Cl 2 The solution was added dropwise to the round-bottomed flask within 1 h. After the dropwise addition, the reaction solution was stirred and reacted at room temperature for 3.5 h. Then filter and filter with CH 2 Cl 2 The solid was washed to obtain a white solid. After the filtrate was spin-dried, it was washed with ether to obtain 7-oxo-bicyclo[2.2.1]heptane-5,6-dibromoic anhydride. Finally, add 7-oxo-bicyclo[2.2.1]heptane-5,6-dibromoic anhydride (1 mmol) and amine compound (0.5 mmol) to the Schlenk containing 2 mL of acetone, and stir at room temperature After 10 hours, TLC spot plate monitoring, after the reaction, filtered, washed with acetonitrile and dried by infrared. Yiel...
Embodiment 3
[0029]
[0030] with 5 mL of CH 2 Cl 2 Add maleic anhydride (10 mmol, 1 g) and freshly distilled furan (20 mmol, 1.4 g) to a 25 mL round bottom flask, stir at room temperature for 24 h, filter, wash with ether, and spin dry. Afterwards, under the protection of nitrogen, the bromine CH 2 Cl 2 The solution was added dropwise to the round-bottomed flask within 1 h. After the dropwise addition, the reaction solution was stirred and reacted at room temperature for 3.5 h. Then filter and filter with CH 2 Cl 2 The solid was washed to obtain a white solid. After the filtrate was spin-dried, it was washed with ether to obtain 7-oxo-bicyclo[2.2.1]heptane-5,6-dibromoic anhydride. Finally, add 7-oxo-bicyclo[2.2.1]heptane-5,6-dibromoic anhydride (1 mmol) and amine compound (0.5 mmol) to the Schlenk containing 2 mL of acetone, and stir at room temperature 1s, TLC plate monitoring, after the reaction, filtered, washed with acetonitrile and dried by infrared. Yield 84%.
[0031] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com