Ta/W mixed heteropoly acid, preparation method and application thereof in acid catalysis and proton conduction
A technology of heteropolyacids and heteropolyanions, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Single crystal structure and other issues, to achieve the effect of strong proton conductivity and high acid catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of Ta / W mixed heteropolyacid
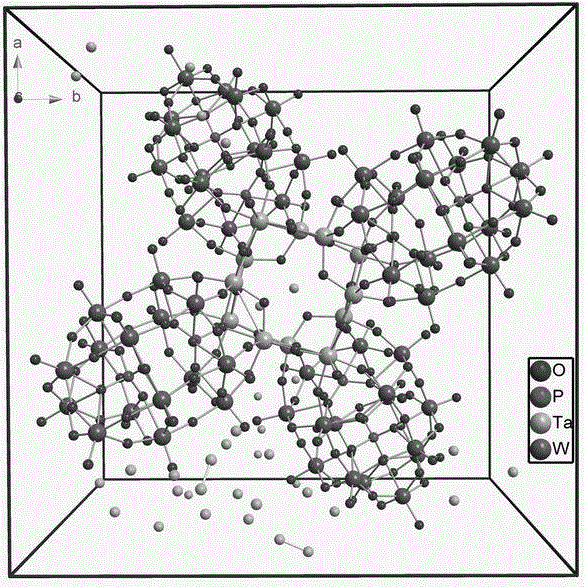
[0024] 1. Precursor mixture H 4 K 8 Na 8 [P 8 W 60 Ta 12 (H 2 O) 4 (OH) 8 o 236 ]·nH 2 O, marked as 1, synthesized according to the method described in the authorized patent (ZL201210236244.0) and literature reports (J. Am. Chem. Soc., 2012, 134, 19716−19721);
[0025] 2. Put 100g of activated cation exchange resin (Amberlite IR120B NA) into a chromatographic column with an inner diameter of 15mm, then pour 400mL of hydrochloric acid solution with a molar concentration of 1mol / L, and control the output rate to 1 drop / 2s. The chromatography column is acidic, then wash the chromatography column with deionized water to neutrality;
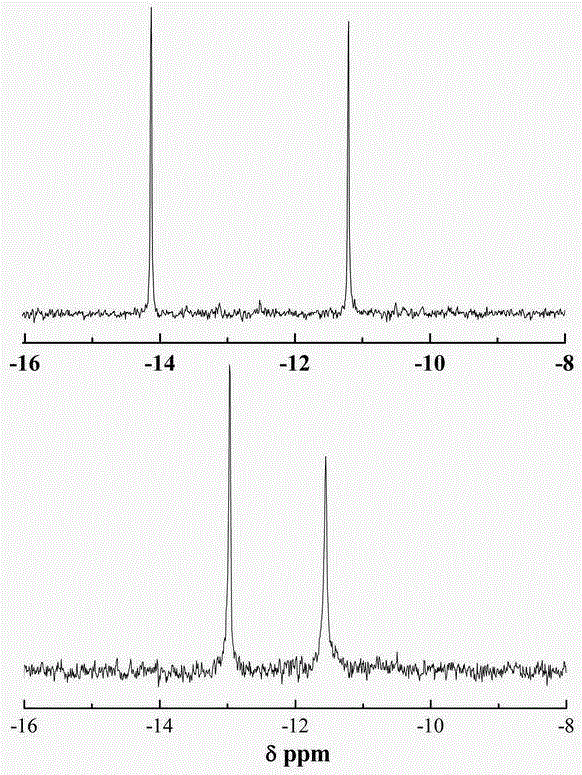
[0026] 3. Dissolve 3g of the precursor 1 obtained in step 1 in 5.0mL deionized water, then pour the above solution into the chromatographic column treated in step 2, control the output rate to 1 drop / 2s, and then use deionized water The chromatographic column was washed to neutrality, and t...
Embodiment 2
[0031] Acid catalytic activity test
[0032] In order to verify the acid catalytic activity of H-1, this example uses H-1 as a catalyst to catalyze the reaction of benzaldehyde with five alcohols. The specific method is as follows: take 3.3mmol of benzaldehyde, 25mmol of alcohol and 10mg of H-1, react in a reaction vessel for 90min, wherein the reaction temperature of reactions 5 and 9 is 100°C, and react 1, 2, 3, 4, 6, 7 and The reaction temperature of 8 is the reflux temperature, and the conversion rate of each reaction is shown in Table 1 by GC-MS after the reaction is finished.
[0033] Table 1 Reaction of benzaldehyde with various alcohols using H-1 as catalyst
[0034] alcohol product Conversion rate (%) a
[0035] Taking the reaction of benzaldehyde and ethylene glycol as an example, when using different heteropolyacids as catalysts, H-1 showed the highest catalytic activity. The specific method is as follows: 3.3mmol of benzaldehyde, 25mmol of ethyle...
Embodiment 3
[0040] Proton conductivity test
[0041] Take some of the prepared samples H-1, press them into thin slices with a thickness of 1 mm and a diameter of 1 cm with a tablet press, sandwich them into the loop of an electrochemical workstation, and test their conductivity under different humidity and temperature.
[0042] Under the conditions of temperature of 25°C and relative humidity of 30%, the electrical conductivity is 7.2×10 -3 S cm -1 . With the increase of relative humidity, the conductivity at 25°C gradually increases, and at 98% relative humidity, the conductivity reaches 5.0×10 - 2 S cm -1 . Such as Figure 5 As shown, in the case of keeping the relative humidity constant at 30%, the conductivity of H-1 increases with the increase of temperature within the tested temperature range (30°C, 45°C, 60°C, 75°C and 95°C). increase, reaching 7.2×10 at 95°C -2 S cm -1 . According to the calculation of Arrhenius curve, the electrical activation energy of H-1 proton con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com