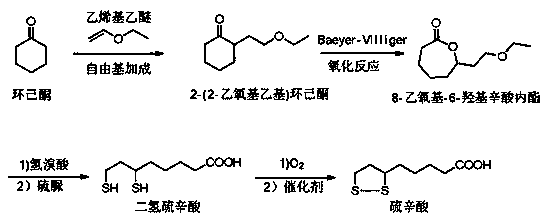
A kind of synthetic method of lipoic acid intermediate
A synthetic method and intermediate technology, applied in the field of preparation of lipoic acid intermediate 2-cyclohexanone, can solve the problems of high cost, high energy consumption, low purity, etc., achieve low cost, high product purity, and reduce distillation steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
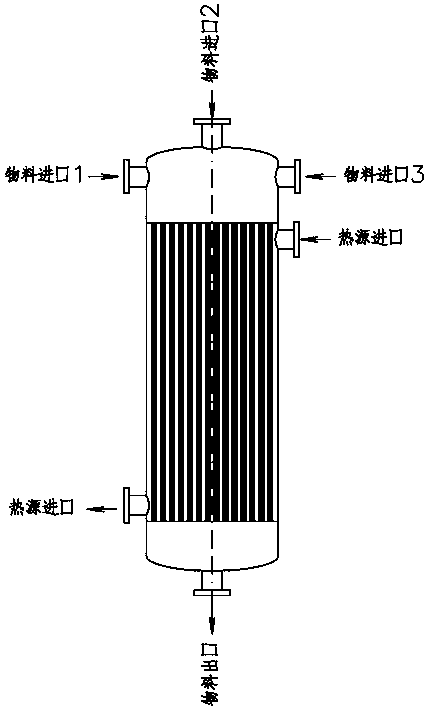
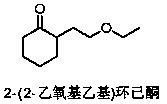
[0025] Add cyclohexanone (50.0kg, 0.51mol) from material inlet 1, add the mixed vinyl ethyl ether (36.8kg, 0.51mol) and hydrogen peroxide (1.7kg, 0.05mol) from material inlet 2, and import 3 Feed 15 mg / L of gaseous ozone, continuously mix and react in the reactor, keep the reaction temperature at 90°C, and keep the residence time for 2 hours, collect the product at the material outlet of the tubular reactor to obtain 2-(2-ethoxyethyl ) Cyclohexanone 76.4kg, purity 96.3%, yield 88.0%.
Embodiment 2
[0027] Add cyclohexanone (50.0kg, 0.51mol) from material inlet 1, add the mixed vinyl ethyl ether (29.6kg, 0.41mol) and hydrogen peroxide (1.7kg, 0.05mol) from material inlet 2, and import 3 Feed 13 mg / L of gaseous ozone, continuously mix and react in the reactor, keep the reaction temperature at 100°C, and keep the residence time for 1.5 hours, collect the product at the material outlet of the tubular reactor to obtain 2-(2-ethoxyethyl ) Cyclohexanone 57.9kg, purity 95.5%, yield 82.9%.
Embodiment 3
[0029] Add cyclohexanone (50.0kg, 0.51mol) from material inlet 1, add the mixed vinyl ethyl ether (40.5kg, 0.56mol) and hydrogen peroxide (1.7kg, 0.05mol) from material inlet 2, and import 3 Feed 14mg / L of gaseous ozone, continuously mix and react in the reactor, keep the reaction temperature at 110°C, and keep the residence time for 1 hour, collect the product at the material outlet of the tubular reactor to obtain 2-(2-ethoxyethyl ) Cyclohexanone 73.8kg, purity 96.1%, yield 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com