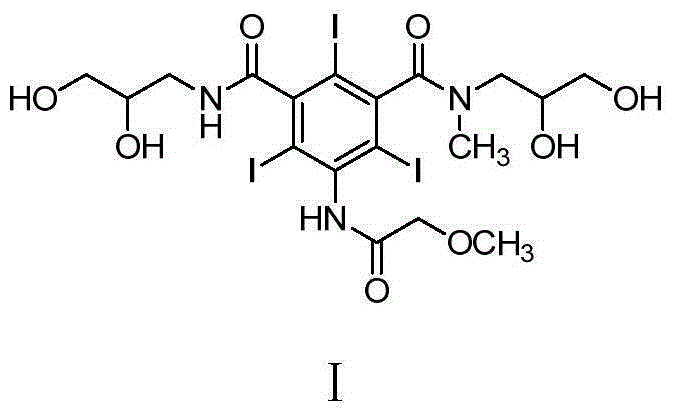
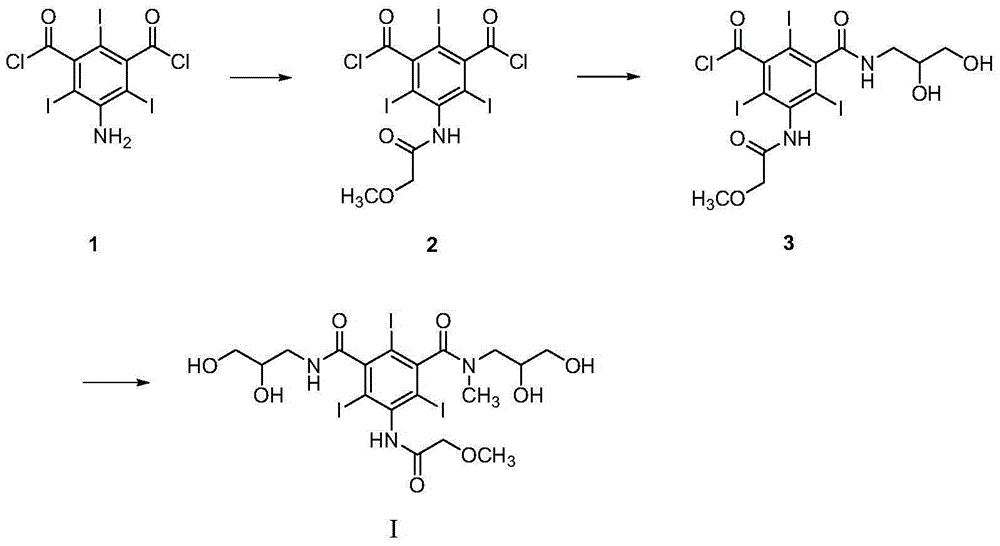
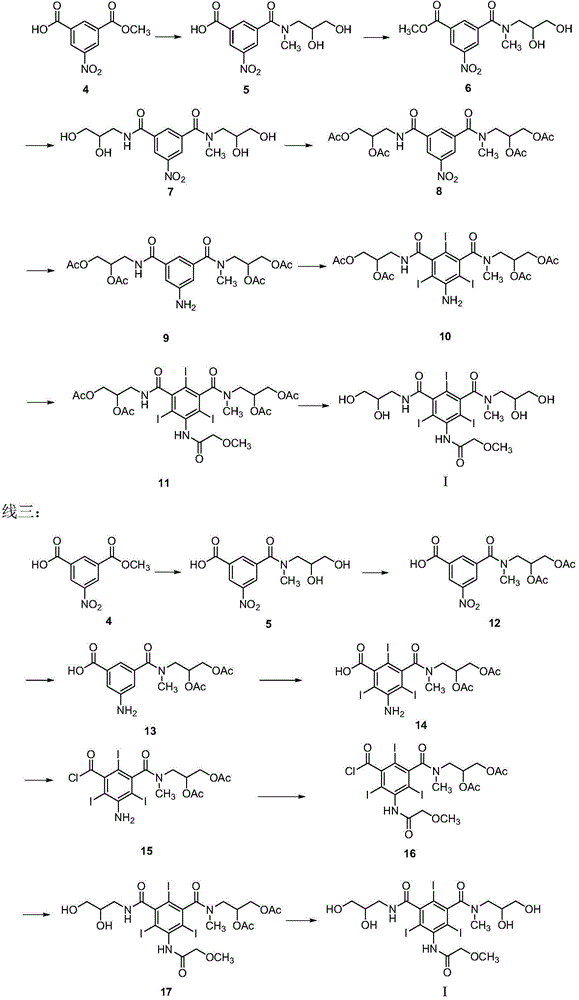
Preparation method and intermediates of iopromide
A technology of iopromide and dimethylacetamide, which is applied to the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of increasing costs, reducing the purity of intermediates and final products, and acetyl protecting groups Easy to be taken off, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: 3-((2,3-dihydroxypropyl)carbamoyl)-5-nitrobenzoic acid methyl ester (formula VII-1)
[0081]
[0082]Add 3-methoxycarbonyl-5-nitrobenzoic acid (formula VIII) (50 g, 0.222 mol) into the reaction flask, add 400 mL of dichloromethane to dissolve it, add dropwise oxalyl chloride (28.5 mL, 0.335 mol) at room temperature, Continue to stir for 1-2 hours after the dropwise addition, then concentrate the reaction liquid, redissolve the concentrated liquid with dichloromethane (125 mL), cool the reaction liquid to below 0°C, add dropwise 3-amino-1,2-propanediol ( 48.5g, 0.53mol), the whole dropwise addition process was maintained for 4 hours, stirred for 0.5 hours after the dropwise addition was completed, the reaction solution was washed with water, the organic layer was dried with anhydrous sodium sulfate, filtered, and concentrated to obtain 3-((2,3-di Hydroxypropyl)carbamoyl)-5-nitrobenzoic acid methyl ester (formula VII-1).
[0083] MS m / z[ESI]:298.1[M+1] ...
Embodiment 2
[0084] Embodiment two: N 1 ,N 3 -Bis-(2,3-dihydroxypropyl)-N 1 -Methyl-5-nitroisophthalamide (Formula VI)
[0085]
[0086] The 3-((2,3-dihydroxypropyl)carbamoyl)-5-nitrobenzoic acid methyl ester (formula VII-1) (29.8g, 0.1mol) obtained in Example 1, 3-methylamino -1,2-Propanediol (15g, 0.14mol) was dissolved in DMF (30mL), reacted at 70-80°C for 5h, and the HPLC purity of the reaction solution was ≥95%.
Embodiment 3
[0087] Example 3: Methyl 3-((2,3-dihydroxypropyl)methylcarbamoyl)-5-nitrobenzoate (Formula VII-2)
[0088]
[0089] Add 3-methoxycarbonyl-5-nitrobenzoic acid (formula VIII) (50 g, 0.222 mol) into the reaction flask, add 400 mL of dichloromethane to dissolve it, add dropwise oxalyl chloride (28.5 mL, 0.335 mol) at room temperature, Continue to stir for 1-2 hours after the dropwise addition, then concentrate the reaction solution, redissolve the concentrate with dichloromethane (125mL), cool the reaction solution below 0°C, and add 3-methylamino-1,2-propanediol dropwise (55.7g, 0.53mol), the whole dropwise addition process was maintained for 4 hours, and after the dropwise addition, stirred for 0.5 hour and the reaction ended, the reaction solution was washed with water, and the organic layer was dried with anhydrous sodium sulfate, filtered, and concentrated to obtain 3-((2, Methyl 3-dihydroxypropyl)methylcarbamoyl)-5-nitrobenzoate (Formula VII-2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com