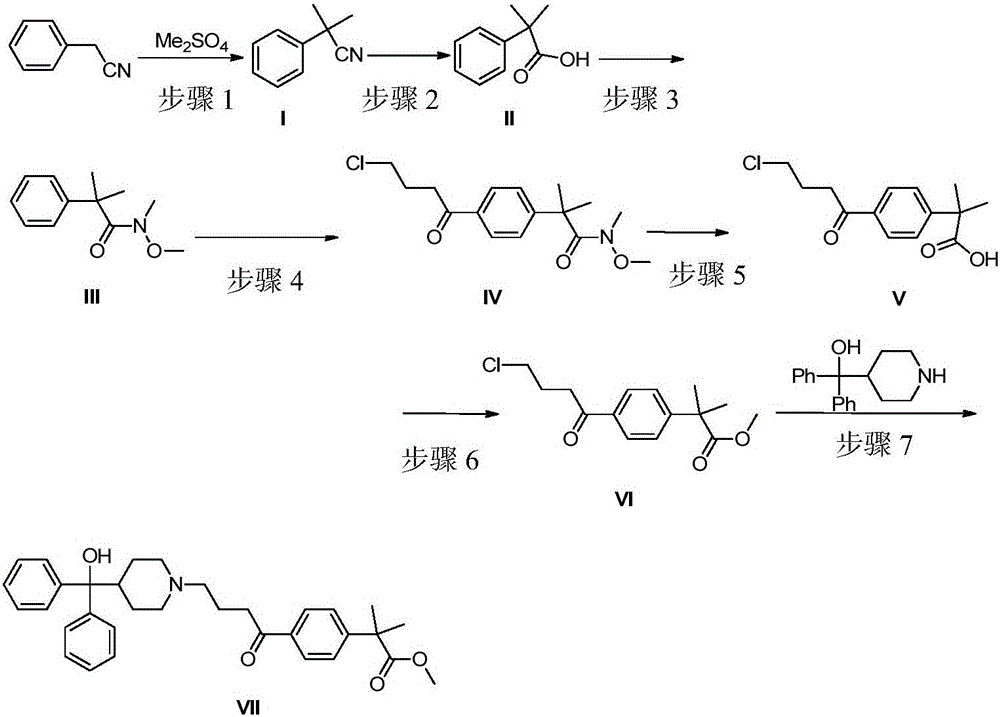
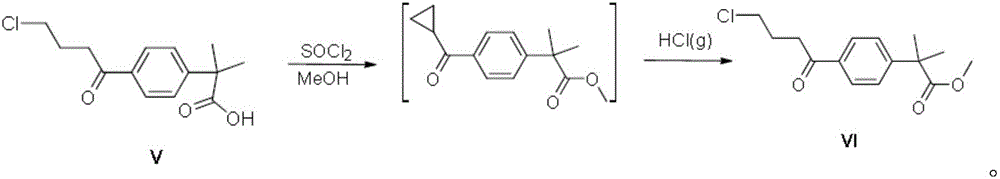
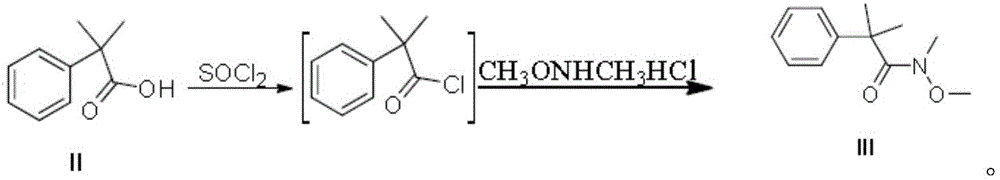Synthetic method of fexofenadine intermediate
A fexofenadine and synthetic method technology, applied in the field of organic synthesis route design and preparation of pharmaceutical intermediates, can solve the problems of condensation influence, difficult separation of impurities, low purity, etc., and achieve the effect of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: In a 1000mL reaction flask, add 50g of phenylacetonitrile, 140g of potassium hydroxide and 250mL of dimethyl sulfoxide, stir at room temperature for 1 hour, add 132g of dimethyl sulfate dropwise, and control the temperature at 35-40°C. React for 1 hour under HPLC monitoring; after the reaction is complete, pour the reaction solution into 1000 g of a stirred ice-water mixture, extract 200 mL*2 with dichloromethane, combine the organic phases, wash with water and saturated brine successively, dry over anhydrous sodium sulfate, and concentrate Obtain a red liquid; put the obtained red liquid into a reaction flask, add 250ml of water and 85g of sodium hydroxide in sequence, heat up and reflux for 48h, monitor by HPLC, after the reaction is complete, cool to 20-25°C, and dichloromethane 100mL*2 Extract the impurities, discard them, acidify the aqueous layer with concentrated hydrochloric acid to pH 1-2, and precipitate a large amount of solids, filter, wash with wa...
Embodiment 2
[0034] Example 2: In a 1000mL reaction flask, add 50g of phenylacetonitrile, 102.4g of sodium hydroxide and 250mL of dimethyl sulfoxide, stir at room temperature for 1 hour, add 118.3g of dimethyl sulfate dropwise, and control the temperature at 35-40°C. React at this temperature for 1 hour, and monitor by HPLC; after the reaction is complete, pour the reaction solution into 1000 g of stirred ice-water mixture, extract 200 mL*2 with dichloromethane, combine the organic phases, wash with water and saturated brine in sequence, and dry over anhydrous sodium sulfate , concentrated to obtain a red liquid; put the obtained red liquid into a reaction flask, add 250ml of water and 85g of sodium hydroxide in turn, heat up and reflux for 48h, monitor by HPLC, after the reaction is complete, cool to 20-25°C, and dichloromethane 100mL *2 extract impurities, discard them, acidify the aqueous layer with concentrated hydrochloric acid to pH 1-2, precipitate a large amount of solids, filter, w...
Embodiment 3
[0035]Example 3: In a 1000mL reaction flask, add 50g of phenylacetonitrile, 95.8g of potassium hydroxide and 250mL of dimethyl sulfoxide, stir at room temperature for 1h, add 161.5g of dimethyl sulfate dropwise, control the temperature at 35-40°C, complete the addition, and React at this temperature for 1 hour, and monitor by HPLC; after the reaction is complete, pour the reaction solution into 1000 g of stirred ice-water mixture, extract 200 mL*2 with dichloromethane, combine the organic phases, wash with water and saturated brine in sequence, and dry over anhydrous sodium sulfate , concentrated to obtain a red liquid; put the obtained red liquid into a reaction flask, add 250ml of water and 85g of sodium hydroxide in turn, heat up and reflux for 48h, monitor by HPLC, after the reaction is complete, cool to 20-25°C, and dichloromethane 100mL *2 extract impurities, discard them, acidify the aqueous layer with concentrated hydrochloric acid to pH 1-2, precipitate a large amount ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com