Bromocarbanine compound and its preparation method and application
A technology of bromocribanine and compound, applied in the field of medicine, can solve the problems of difficulty in finding antiarrhythmic drugs, hypothyroidism or hyperthyroidism, lung damage, adverse reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
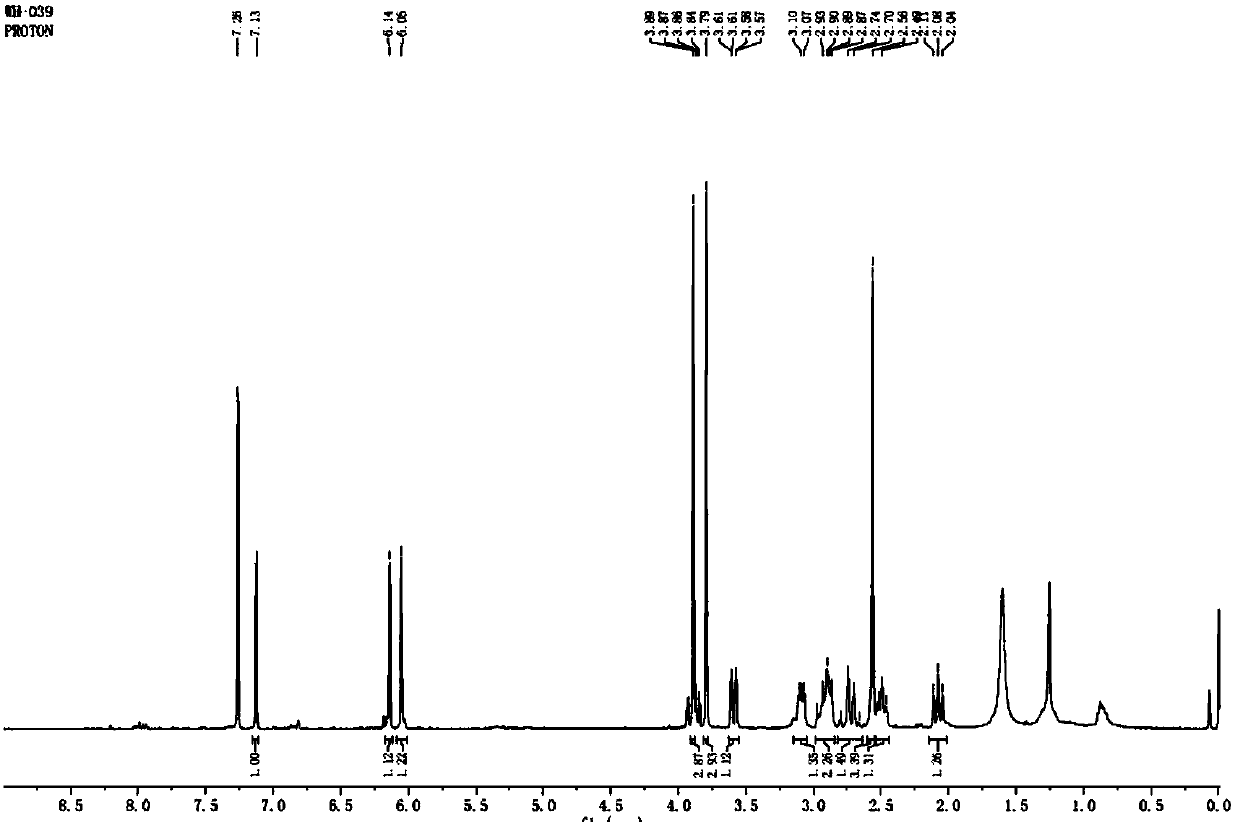
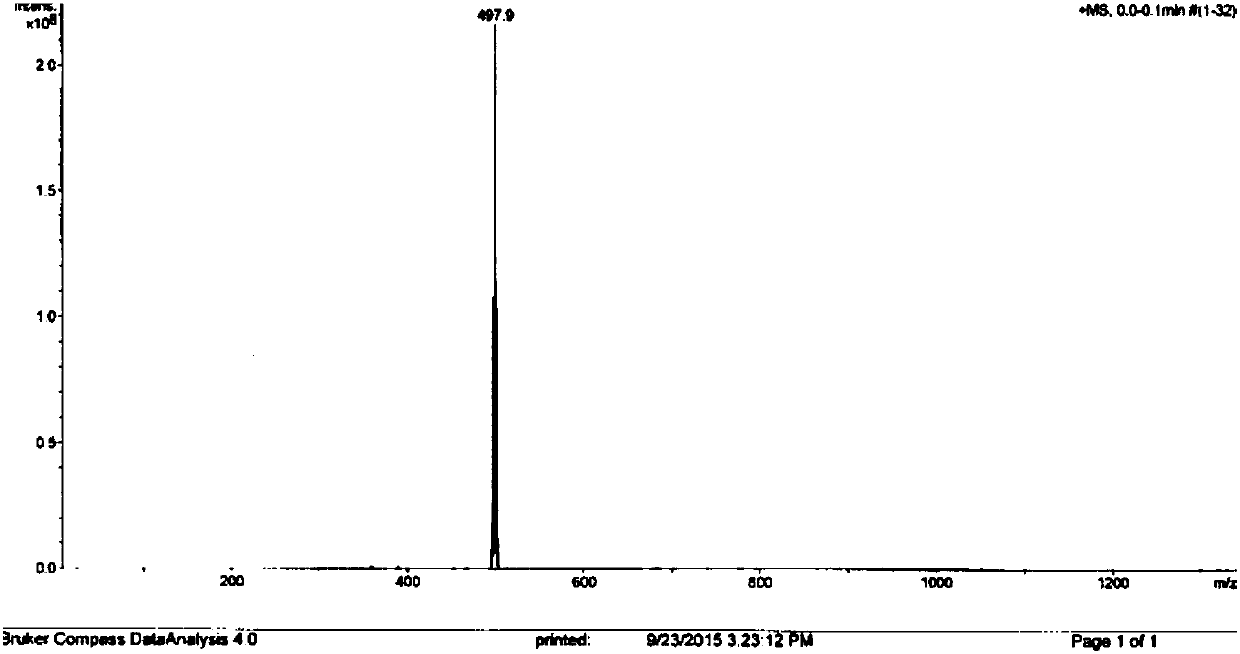
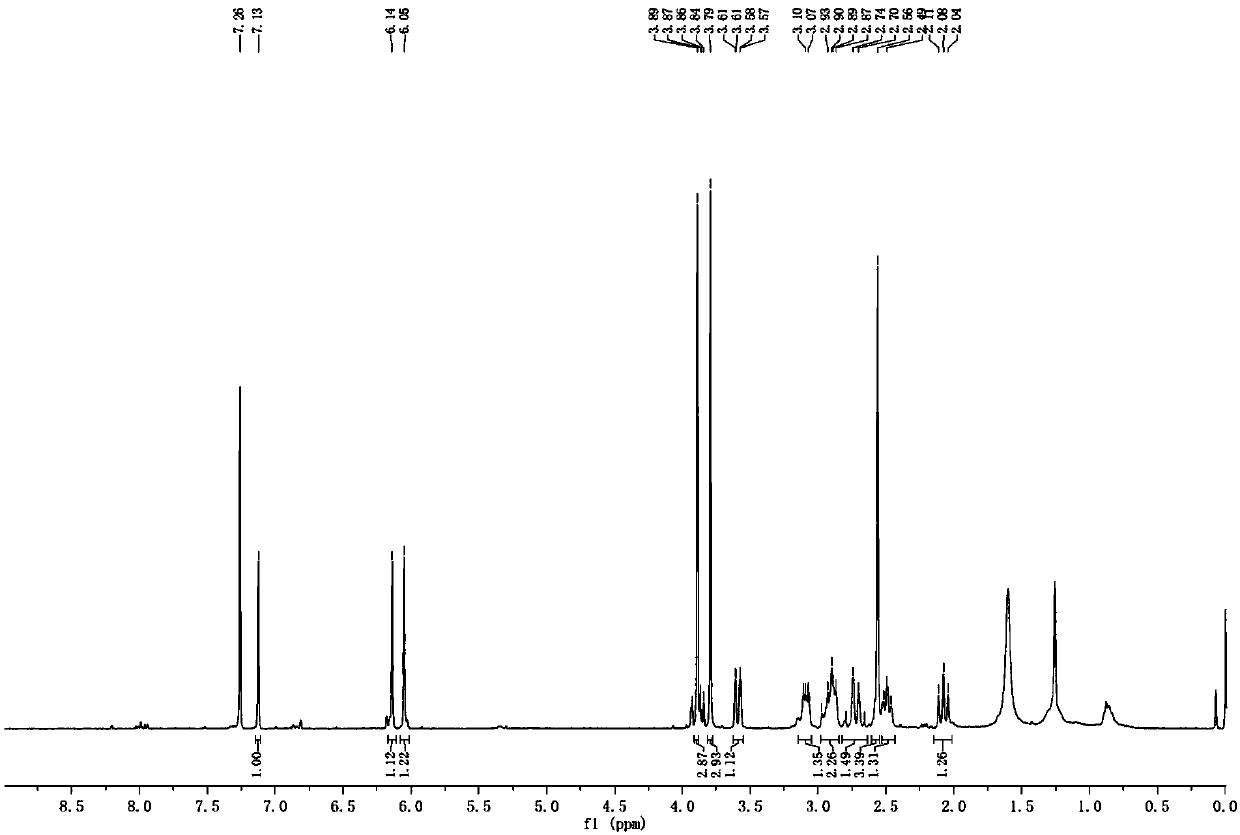
Image
Examples
Embodiment 1
[0084] Step (1), under ice-bath condition, add trifluoroacetic acid to make grambanine dissolve completely in grambanine; Then slowly add N-bromosuccinimide, N-bromosuccinimide The addition time of the solution was more than 10 minutes; after the addition, the ice bath was removed, and the reaction solution was stirred at room temperature for 11 hours, and then rotary evaporated to remove the solvent trifluoroacetic acid to obtain a concentrate;
[0085] The mass volume ratio of kebanine to trifluoroacetic acid is 0.2:4.9; the molar ratio of kebanine to N-bromosuccinimide is 1:1.1;
[0086] Step (2), add water that is 9.5 times of the volume of trifluoroacetic acid in the concentrate that step (1) obtains, then use saturated Na 2 CO 3 The aqueous solution was adjusted to pH 8, followed by CH 2 Cl 2 Perform the extraction, extracting 2 times, each extraction of CH 2 Cl 2 The amount used is one-fifth of the volume of water, and the organic layers are combined;
[0087] Ste...
Embodiment 2
[0113] An antiarrhythmic drug, comprising the following components in parts by weight: 250 parts of bromocarbanine compounds, 60 parts of powdered sugar, and 5 parts of dextrin; the bromocarbanine compounds are 3 parts - Bromocarbanine or 10,11-bromocarbanine and pharmaceutically acceptable salts thereof. The bromocarbanine compound is 10,11-bromocarbanine.
[0114] The preparation method is the same as in Example 1.
Embodiment 3
[0116] An antiarrhythmic drug, comprising the following components in parts by weight: 120 parts of bromocarbanine compounds, 90 parts of powdered sugar, and 10 parts of dextrin; the bromocarbanine compounds are 3 parts - Bromocarbanine or 10,11-bromocarbanine and pharmaceutically acceptable salts thereof.
[0117] The bromocarbanine compound is a pharmaceutically acceptable salt of 3-bromocarbanine.
[0118] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com