Mycobacterium tuberculosis PGL-tb1 oligosaccharide conjugate as well as preparation method and application thereof
A technology of Mycobacterium tuberculosis and a conjugate is applied in the field of tuberculosis vaccine development to achieve the effects of solving the problem of low immune protection, avoiding the problem of bacterial drug resistance and improving the immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The general synthesis method of the above-mentioned Mycobacterium tuberculosis PGL-tb1 oligosaccharide conjugate, the steps are as follows:
[0059] ⑴Reaction between oligosaccharides and linkers
[0060] Dissolve triphenylphosphine (0.07 equivalents), cuprous iodide (0.14 equivalents) and bistriphenylphosphorus palladium dichloride (0.07 equivalents) in triethylamine, and stir the reaction at 40°C for 15 Minutes, configured as a catalyst, ready for use. The prepared oligosaccharide substrate and 6-chlorohexyne (10 equivalents) were dissolved in triethylamine, and then the prepared catalyst was added. The reaction was stirred at 40°C for 2 hours. TLC detection showed that the reaction was complete, and ethyl acetate was added to the reaction solution, extracted with 2mol / L hydrochloric acid solution, then, the organic phase was further extracted with saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the s...
Embodiment 1
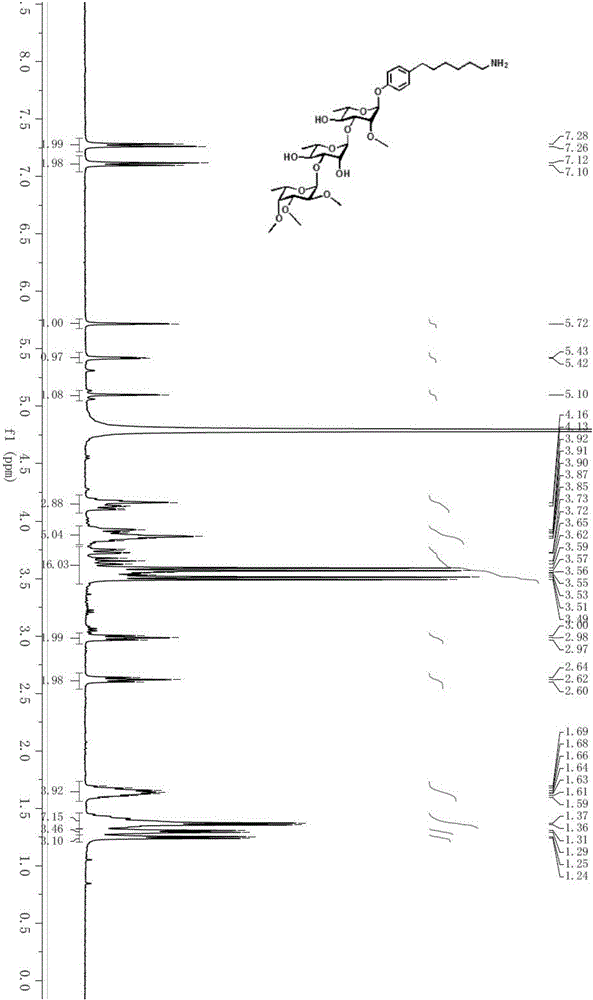
[0069] (2,3,4-Tri-O-methyl-α-L-fucosyl)-(1→3)-α-L-rhamnosyl-(1→3)-(1-(6 -(4-Hydroxyphenyl)-hexylamino)-2-O-methyl-α-L-rhamnose)-CRM 197 Synthesis of the conjugate (PGL-tb1-CRM 197 )Synthesis
[0070] ⑴2,3,4-tri-O-methyl-α-L-fucosyl-(1→3)-2,4-di-O-benzyl-α-L-rhamnosyl-(1 → 3)-1-(1-chloro-6-(4-hydroxyphenyl)-5-hexyne)-2-O-methyl-4-benzyl-α-L-rhamnose (A1) synthesis
[0071]
[0072] Weigh 9.9 mg triphenylphosphine (39 μmol), 14.4 mg cuprous iodide (75 μmol) and 26.4 mg bistriphenylphosphine palladium dichloride (39 μmol) and dissolve them in redistilled triethylamine (18 mL). Stir the reaction at 40°C for 15 minutes, configure it as a catalyst, and set aside. With 2,3,4-tri-O-methyl-α-L-fucosyl-(1→3)-2,4-di-O-benzyl-α-L-rhamnosyl-( 1→3)-1-p-iodophenyl-2-O-methyl-4-benzyl-α-L-rhamnose (1.5g, 0.51mmol, refer to Angew.Chem.Int.Ed.2012,51 : the synthesis method of 11774) and 0.6mL (5.08mmol) of 6-chlorohexyne were dissolved in 6.09mL triethylamine, then, adding catalyst 12...
Embodiment 3
[0084] 2,3,4-Tri-O-methyl-α-L-fucosyl-(1→3)-α-L-rhamnosyl-(1→3)-1-p-phenylhexylamino- Synthesis of 2-O-methyl-α-L-rhamnose-BSA glycoconjugate
[0085]
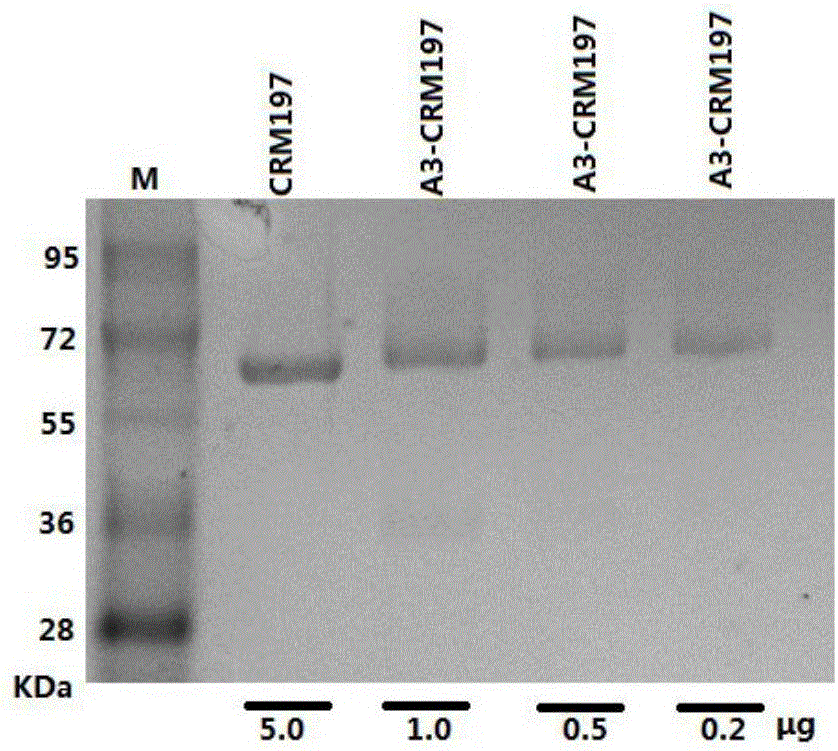
[0086] Take A3 (5mg), BSA (20mg) and synthesize A3-BSA conjugate (23mg) according to the method of step (4) of the general synthesis method. Mass Spectrum: MALDI-TOF-MS (m / z) 66415
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com