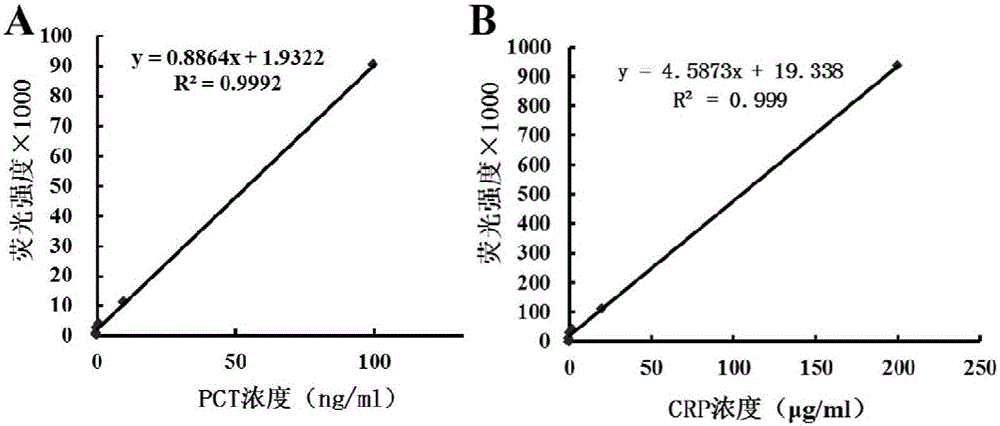
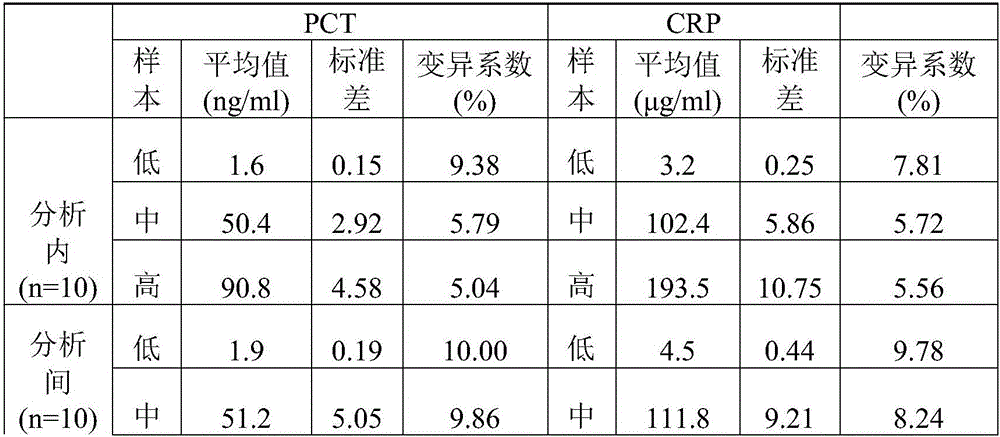
PCT (Procalcitonin) and CRP (C-Reactive Protein) double-label time resolution fluorescence immunoassay method for simultaneously detecting bacterial meningitis and viral meningitis
A viral meningitis and bacterial technology, applied in the biological field, can solve the problems of difficulty in meeting the needs of early diagnosis of meningitis, poor sensitivity, etc., and achieve the effect of shortening the time of disease diagnosis, reducing anxiety and panic, and reducing the molecular weight of antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] 1. Preparation of PCT-CRP recombinant antigen
[0029] PCT and CRP specific sequences are selected and combined to obtain PCT-CRP recombinant antigen, the nucleotide sequence of which is as follows:
[0030] GCCCTGGAAAGCAGCCCGGCCGATCCGGCCACCCTGAGCGAAGATGAAGGCGGCGGCGGCGCCATTGGCGTGGGCGCCCCGGGCAAAAAACGCGATATGAGCAGCGATGGCGGCGGCGGCAAAGCCCCGCTGACCAAAACCGCTGAAAAGCCTTTACCSEGTGTGCCTGCATGGCGGCGGCGCAACTTTGAAGGCAGCCANOGCCTGGTGGGC as nucleotides in GATATTG. The present invention obtains the above-mentioned PCT-CRP recombinant antigen through a large number of preliminary studies and preliminary experiments, through screening and optimizing combination of specific fragments related to PCT and CRP antigens.
[0031]The above-mentioned PCT-CRP recombinant polypeptide was prepared and expressed by genetic engineering technology, its amino acid sequence is: ALESSPADPATLSEDEGGGGAIGVGAPGKKRDMSSDGGGGKAPLTKPLKAFTVCLHGGGGNFEGSQSLVGDIGNVN, and its amino acid sequence is shown in SEQ ID NO:1. ...
Embodiment 1
[0043] Example 1 A PCT and CRP double-labeled time-resolved fluorescent immunoassay kit for simultaneous detection of bacterial and viral meningitis
[0044] 1. Preparation of solid-phase coated antibodies: Dilute PCT and CRP antibodies to 3 μg / ml and 4 μg / ml with 50 mmol / L, pH 9.6 carbonate buffer, respectively, and add 100 μl / well to a 96-well plate , overnight at 4°C, pour off the coating solution, add blocking solution at 200 μl / well, block overnight, pour off the blocking solution, wash and pat dry, and store in a vacuum at -20°C.
[0045] 2. Eu 3+ and Sm 3+ Preparation of labeled antibody: Add 0.5 mg of labeled antibody into a centrifuge tube with filter membrane from Millipore Company, and centrifuge at 8000 r / min for 6 min. Then labeling buffer (50mmol / L, NaCO 3 , pH 9.0) were washed 6 times. 200 μl of labeled antibody and 50 μg of europium (or samarium) labeling reagent were thoroughly mixed and shaken at room temperature overnight.
[0046] 3. Eu 3+ and Sm 3+ ...
Embodiment 2
[0049] Example 2 A PCT and CRP double-labeled time-resolved fluorescent immunoassay method for simultaneous detection of bacterial and viral meningitis
[0050] The present invention uses the kit described in Example 1 to quantitatively detect PCT and CRP levels. The detection operation steps are: add 25 μl of PCT / CRP reference standard or serum to be tested on the coated enzyme-linked plate, and then add 200 μl of analysis buffer solution, incubated with shaking for 1h, washed with washing solution for 4 times, and then added Eu 3+ -PCT monoclonal antibody with Sm 3+ - CRP monoclonal antibody mixture 200 μl / well, incubate with shaking for 1 hour, wash with washing solution 6 times, finally add 200 μl / well of enhancement solution and shake for 5 minutes, then detect on a time-resolved fluorescence detector.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com