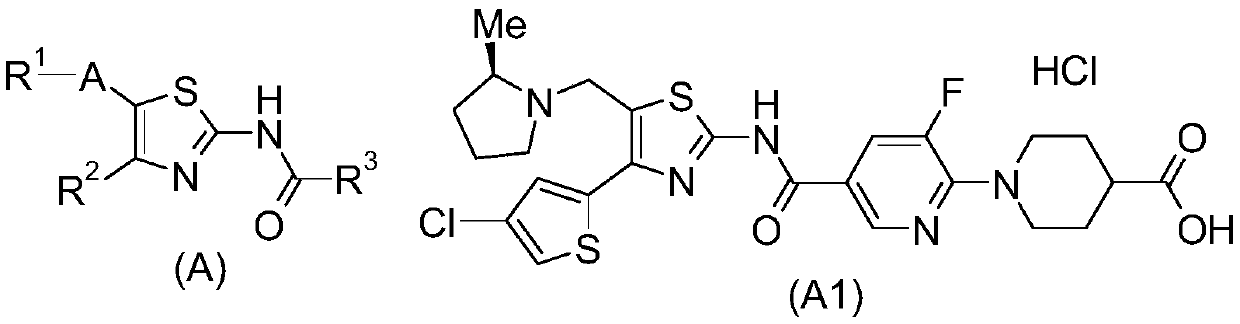
2-Acylaminothiazole derivatives or salts thereof
A technology of thiazole and carbamoyl, applied in the field of 2-acylaminothiazole derivatives or their salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0370] Hereinafter, the production method of the compound of formula (I) will be described in further detail based on the examples. It should be noted that the present invention is not limited to the compounds described in the following examples. In addition, the production method of the raw material compound is shown in the production example. In addition, the production method of the compound of the formula (I) is not limited to the production method of the specific examples shown below, and the compound of the formula (I) can also be produced by a combination of these production methods, or it is obvious to those skilled in the art method of manufacture.
[0371] In addition, in this specification, naming software, such as ACD / Name (registered trademark, Advanced Chemistry Development, Inc.), may be used for naming compounds.
[0372] In the measurement of powder X-ray diffraction, RINT-TTRII was used, and the measurement was carried out under the following conditions: th...
manufacture example 1
[0378] 4-[3-Fluoro-5-(trifluoromethyl)phenyl]-5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-1,3-thiazole-2 - A mixture of amine (1.0 g), 5-chloropyrazine-2-carboxylic acid (685 mg), COMU (1.9 g), dioxane (10 mL) and N,N-diisopropylethylamine (1.5 mL) Stir at room temperature for 1 hour. The reaction mixture was diluted with ethyl acetate, washed with water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain 5-chloro-N-(4-[3-fluoro-5-(trifluoromethyl)phenyl]-5-{[ (2R)-2-Methylpyrrolidin-1-yl]methyl}-1,3-thiazol-2-yl)pyrazine-2-carboxamide (800 mg).
manufacture example 2
[0380] 5-Chloropyrazine-2-carboxylic acid (1.7 g), N,N-dimethyl-4-aminopyridine (340 mg) and WSCD.HCl (2.1 g) were added to 5-{[(2R)-2 -Methylpyrrolidin-1-yl]methyl}-4-[4-(trifluoromethyl)thiophen-2-yl]-1,3-thiazol-2-amine (2.9g) and dichloromethane ( 60 mL) and stirred at 40 °C for 15 minutes. The reaction mixture was cooled to room temperature, diluted with chloroform, and washed with saturated aqueous sodium bicarbonate solution. The aqueous layer was extracted with chloroform / methanol, and the organic layers were combined and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (chloroform-ethyl acetate) to obtain 5-chloro-N-(5-{[(2R)-2-methylpyrrolidin-1-yl]methyl}-4 as a solid -[4-(Trifluoromethyl)thiophen-2-yl]-1,3-thiazol-2-yl)pyrazine-2-carboxamide (2.4 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com