Fluorescent probe for detecting hydrazine and application thereof
A fluorescent probe and compound technology, applied in the field of fluorescent probes for detecting hydrazine, can solve the problems of long response time, affecting hydrazine detection, low probe sensitivity, etc., achieve simple post-processing process, strong anti-interference ability of other ions, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Probe compound N-N 2 h 4 Synthesis of -CN:
[0026]
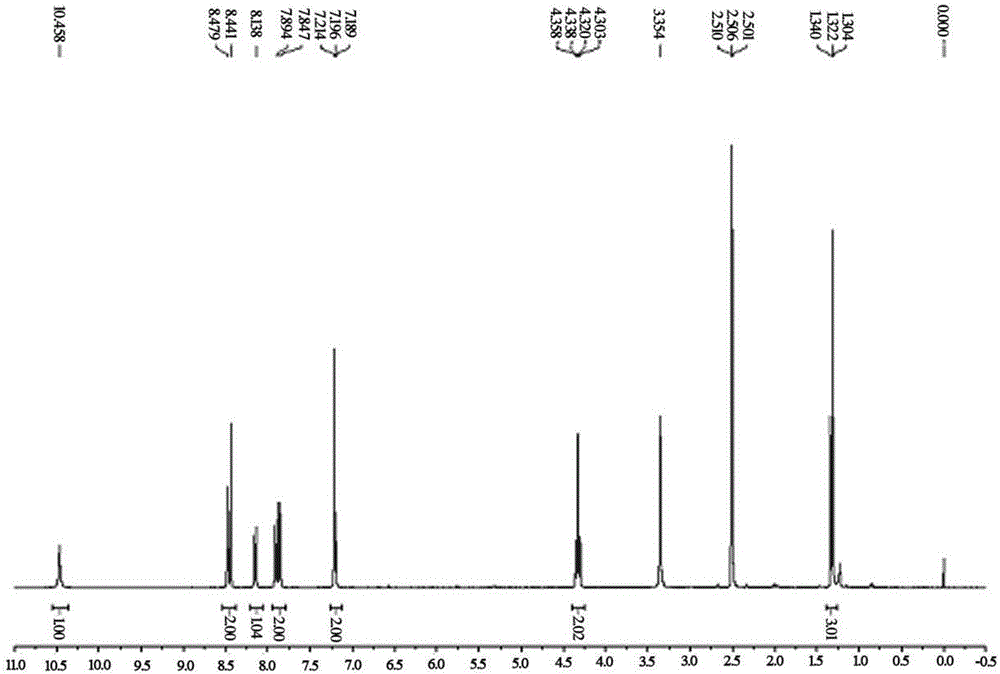
[0027] Compound 1 (0.5mmol, 100.0mg) and compound 2 (0.55mmol, 62.2mg) were added to the reaction flask under a nitrogen atmosphere, and then piperidine (0.55mmol, 46.8mg) and ethanol / acetonitrile (v / v= 1 / 1, 5.0mL) was added to the above reactor at one time, and after reacting at room temperature for 5 hours, the reaction was detected on a plate until the raw materials disappeared, and the probe N-N was obtained by suction filtration 2 h 4 -CN. 1 HNMR (400MHz, DMSO-d 6 ):10.47(s,1H),8.46(d,J=15.2Hz,2H),8.15(dd,J 1 =1.6Hz,J 2 =8.8Hz,1H),7.88(dd,J 1 =10Hz,J 2 =20,2H),7.21(s,1H),7.20(t,J=10,1H),4.33(dd,J 1 =7.2,J 2 =14.4,2H), 1.32(t,J=16,3H).
Embodiment 2
[0029] Probe compound N-N 2 h 4 Absorption spectrum of -CN
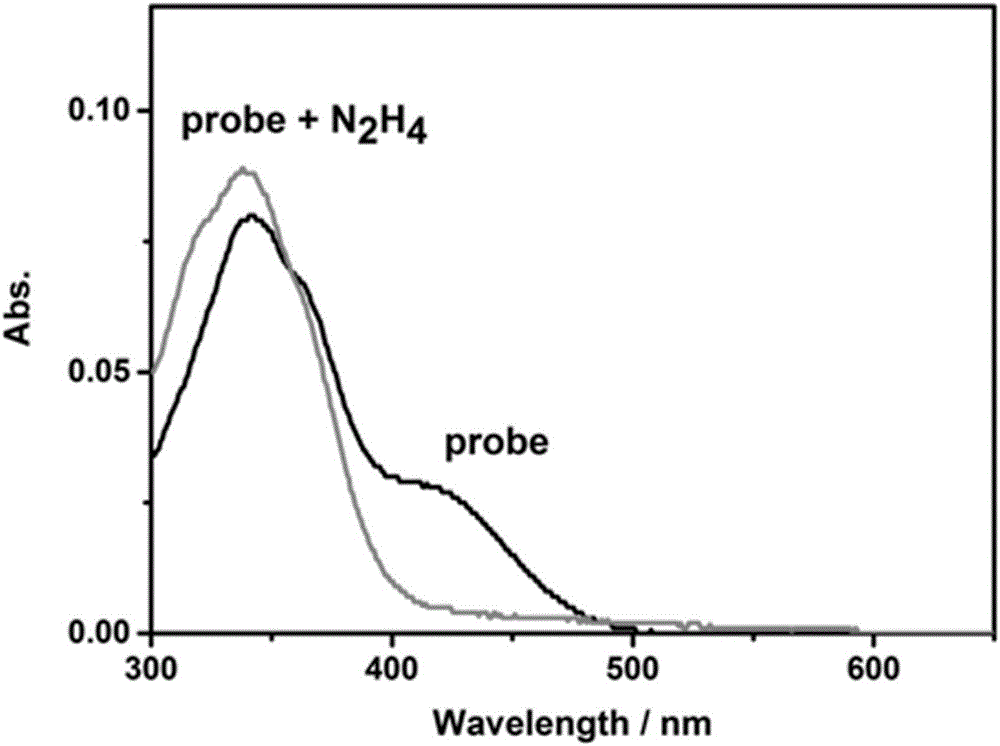
[0030] Get the probe N-N that embodiment 1 prepares 2 h 4 -CN was dissolved in dimethyl sulfoxide (DMSO) to make a 1 mmol / L stock solution. Take two portions of 30 μL from the stock solution, add them to two 5mL centrifuge tubes, dilute to 3mL with a solution of PBS buffer solution (0.1mol / L, pH=7.5) and DMSO at a volume ratio of 1:1, and add 100 equiv to one portion N 2 h 4 The standard solution was not added to the other part, and reacted for 2 hours for absorption test. Such as figure 2 shown. Depend on figure 2 Visible, add N 2 h 4 The absorption maximum of the rear probe is at 340 nm.
Embodiment 3
[0032] Probe compound N-N 2 h 4 -CN with N 2 h 4 The change of fluorescence spectrum with the increase of equivalent
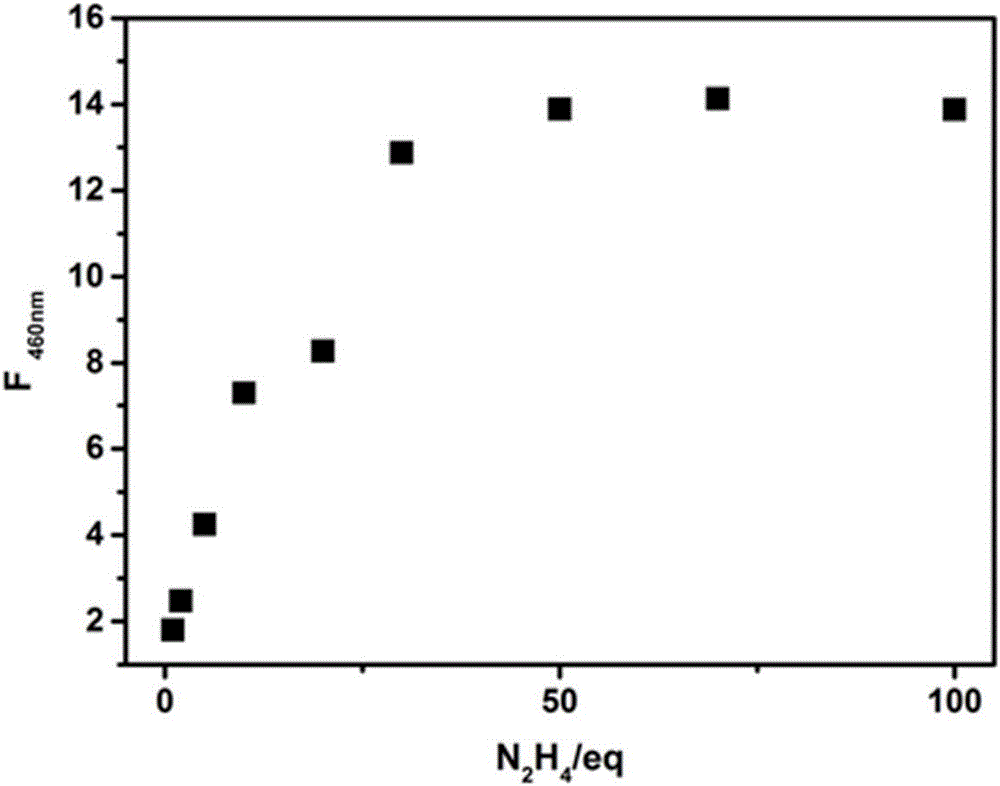
[0033] Get the probe N-N that embodiment 1 prepares 2 h 4 -CN was dissolved in dimethyl sulfoxide (DMSO) to make a 1 mmol / L stock solution. Take 30μL from the stock solution and add it to a 5mL centrifuge tube, add different equivalents (0-100equiv) of N 2 h 4 The standard solution was diluted to 3mL with PBS buffer solution (0.1mol / L, pH=7.5) and DMSO at a volume ratio of 1:1, and its fluorescence properties were measured. Fluorescence spectra such as image 3 shown. Depend on image 3 It can be seen that with N 2 h 4 The fluorescence gradually increases with the addition of equivalents, when adding 50 equivalents of N 2 h 4 When , the fluorescence of the system reaches saturation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com