A mitochondrial-targeted nitrosyl hydrogen molecular fluorescent probe and its preparation method and application
A nitrosyl hydrogen and fluorescent probe technology, applied in the field of analytical chemistry, can solve problems such as DNA chain damage and body damage, and achieve the effects of good selectivity, easy synthesis, and strong resistance to other molecular interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
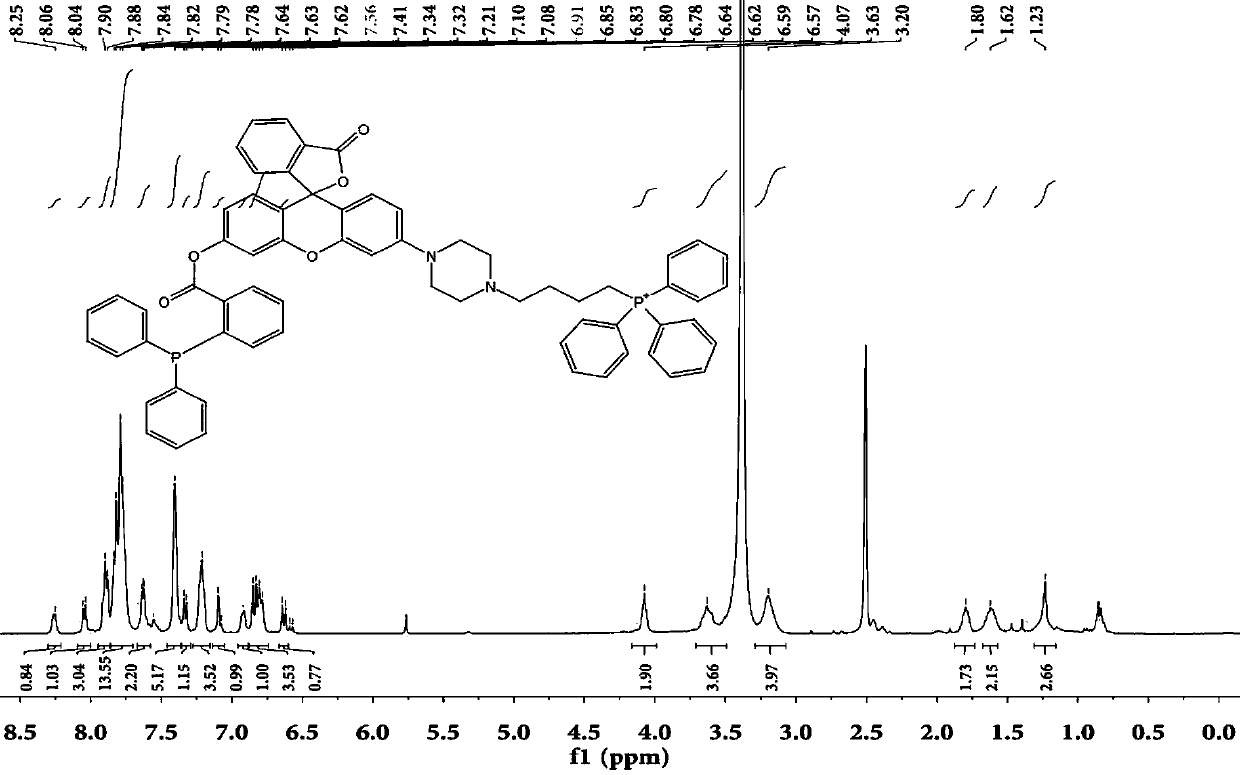
[0038] Synthesis of target probe Mito-HN
[0039] 1) Synthesis of compound MHN-1:
[0040]
[0041] Compound 2′,4′-dihydroxy-2-phenonebenzoic acid (1.0g, 3.88mmol, 1eq) and m-hydroxyphenylpiperazine (758.3mg, 4.26mmol, 1.1eq) were dissolved in 20mL of trifluoroacetic acid, Heat to reflux at 90°C and react overnight. Use a TLC plate to detect the reaction. After the reaction is complete, spin dry the solvent trifluoroacetic acid under reduced pressure, dissolve it with 1ml ethyl acetate, and separate it with a silica gel column. The silica gel particle size is 200-300 mesh, and the eluent ratio is methanol / di Chloromethane=1:5. The yield was 81%.
[0042] 2) Synthesis of compound MHN-2:
[0043]
[0044] Compound MHN-2 (1.1g, 2.74mmol, 1eq), NaHCO 3(691mg, 8.22mmol, 3eq) was dissolved in 20mL of acetonitrile and reacted at room temperature for 10min, then added Fmoc-Cl (850mg, 3.29mmol, 1.2eq), under nitrogen protection, reacted at room temperature for 3h, detected t...
Embodiment 2
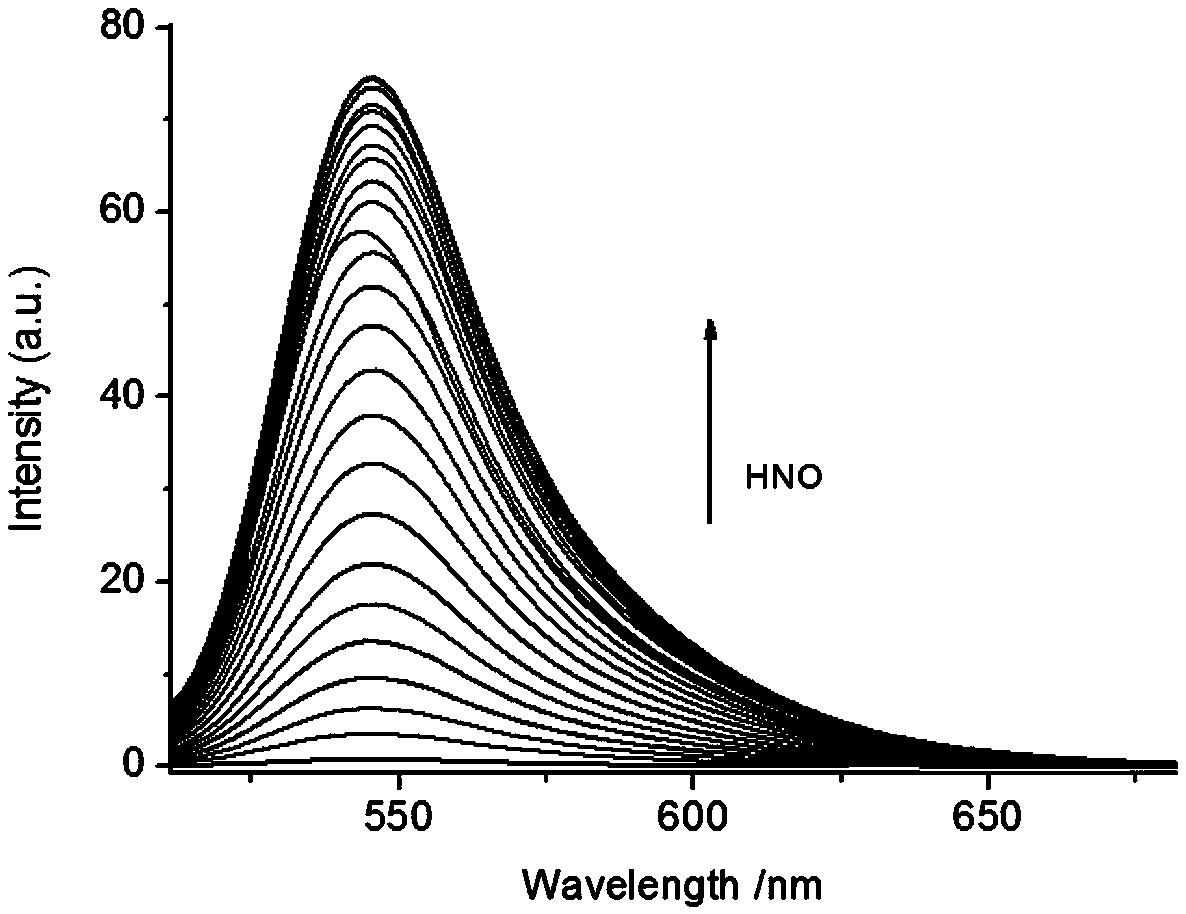
[0055] Changes of the fluorescence spectrum of the fluorescent probe Mito-HN with the increase of HNO addition
[0056] The Mito-HN fluorescent hydrogen nitrosyl probe prepared in Example 1 was dissolved in N,N-dimethylformamide (DMF) to prepare a 1 mmol / L stock solution. Take 30 μL from the stock solution and add it to a 5 mL centrifuge tube, dilute it with 2 mL of PBS (0.1 mol / L, pH=7.4), and then add different equivalents (0-10 eq) of AS salt (HNO donor) standard solution, Dilute it to 3mL with PBS buffer solution, and measure its fluorescence properties with 450nm as excitation light. Fluorescence spectra such as figure 2 shown. Depend on figure 2 It can be seen that the fluorescence at 545nm increases gradually with the increase of the amount of HNO added.
Embodiment 3
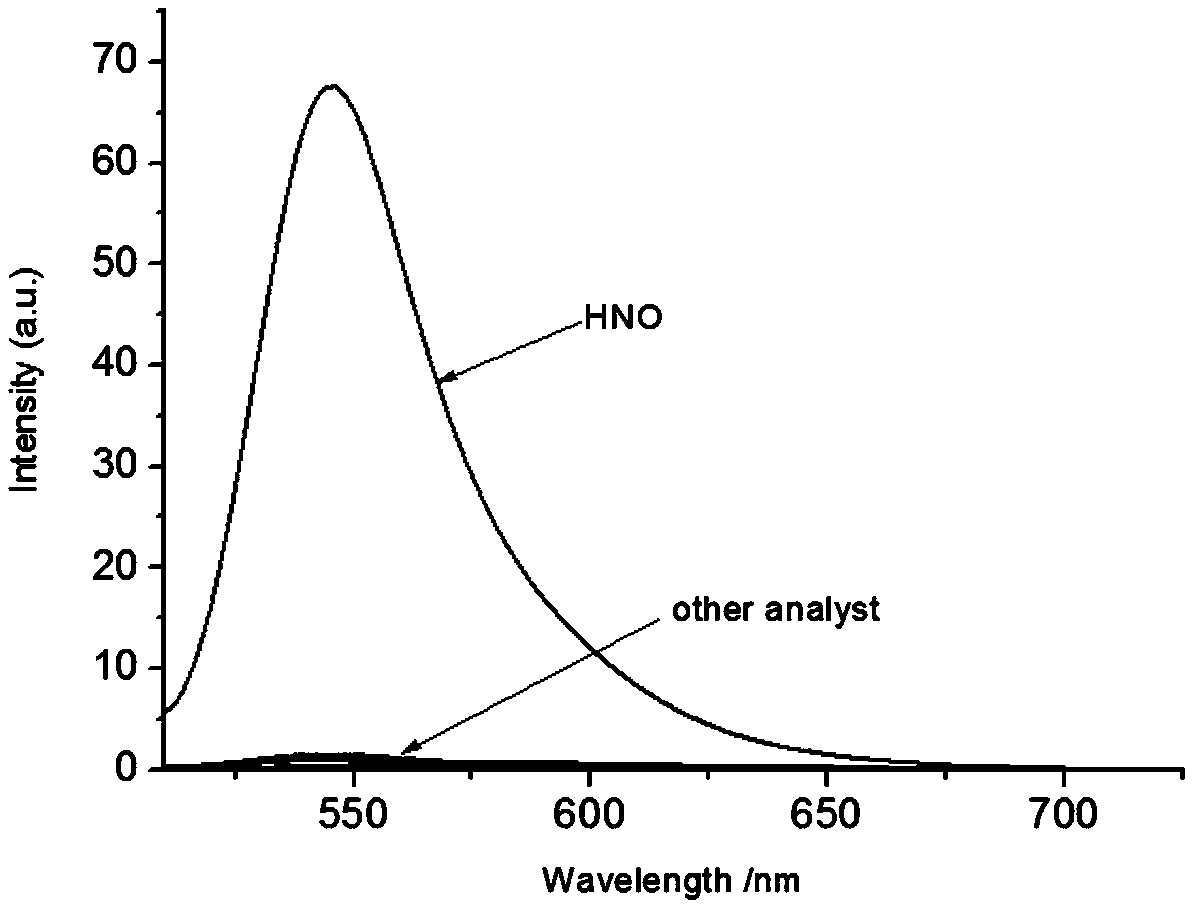
[0058] The selectivity of compound Mito-HN nitrosyl hydrogen fluorescent probe to different molecules or ions
[0059] Take 30 μL from the fluorescent probe stock solution in Example 2 and add them to 18 5mL centrifuge tubes respectively. After diluting with 2mL PBS, add equimolar amounts of competing molecular standard solutions to 16 centrifuge tubes respectively, one of which Do not add any ions, as a blank sample, and add an equimolar amount of HNO standard solution to the other, and detect the change of the fluorescence emission spectrum of the solution after 15 minutes, with 450nm as the excitation light, the results are as follows: image 3 and Figure 4 shown. Depend on image 3 and Figure 4 It can be found that other metal ions, reducing compounds, oxidizing compounds, etc. have almost no effect on the fluorescence of compound Mito-HN at 545nm, but the addition of nitrosyl hydrogen solution can significantly enhance the fluorescence of compound Mito-HN at 545nm. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com