Drug carrier with end group modified by hydrogen sulfide fluorescent probe as well as preparation and application thereof
A fluorescent probe, hydrogen sulfide technology, used in drug combinations, preparations for in vivo tests, medical preparations with inactive ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the synthesis of the initiator 1 of atom transfer radical polymerization:
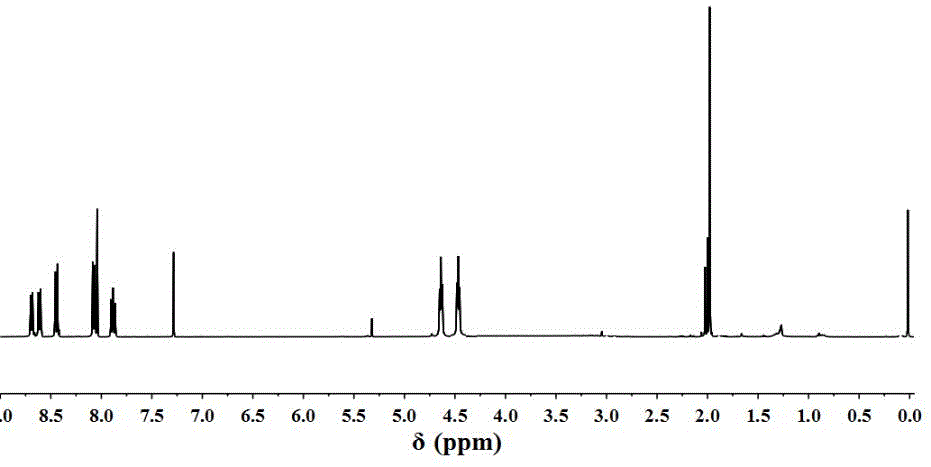
[0049] Dissolve 0.64g (2.0mmol) of 4-bromo-N-hydroxyethyl-1,8-naphthalimide in dimethylformamide, cool to 0°C, and then add 0.052g (2.4mmol) of bromoisobutyryl Bromine was added dropwise to the above solution, and reacted at room temperature for 12 hours after the dropwise addition. Use a TCL plate to detect the disappearance of the raw material 4-bromo-N-hydroxyethyl-1,8-naphthalimide point, and stop the reaction. Precipitate the reaction solution in deionized water, dissolve the precipitate with dichloromethane, extract and wash 3 times with deionized water, dry over sodium sulfate without deionized water, spin dry, and separate with a silica gel column. The particle size is 200-300 mesh, the eluent ratio is ethyl acetate / petroleum ether=1:10, and the yield is 86%. figure 1 for initiator 1 1 HNMR diagram, 1 H-NMR (400MHz, CDCl 3 )δ8.64(d,J=7.2Hz,1H,Ar-H),8.61(d,J=8.4Hz,1H,Ar...
Embodiment 2
[0050] Embodiment 2: the synthesis of macromolecular atom transfer radical polymerization initiator 2:
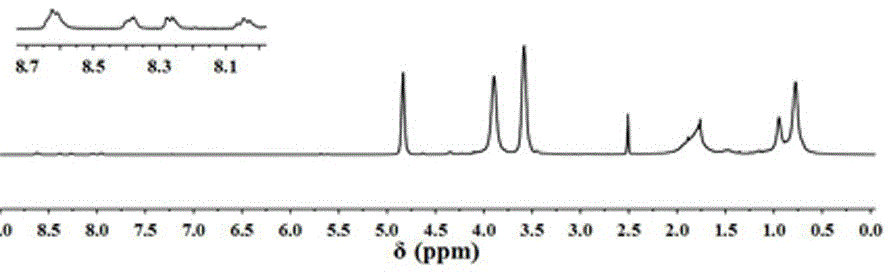
[0051] Dissolve 0.469g (1.0mmol) of initiator 1, 5.2g (40mmol) of hydroxyethyl methacrylate and 0.173g (1.0mmol) of pentamethyldiethylenetriamine in 8ml of dimethylformamide and place in a reaction flask ; Use nitrogen bubbling method to remove oxygen for 30 minutes at room temperature, add 0.144 g (1.0 mmol) cuprous bromide under nitrogen protection, and then pass nitrogen for 15 minutes, seal the reaction bottle, and react at room temperature for 6 hours. The reaction was terminated by exposing the reaction solution to air. After diluting the reaction liquid with 30ml of tetrahydrofuran, the copper ions were removed through a neutral aluminum oxide column, and the eluent ratio was absolute ethanol / tetrahydrofuran=1:40. After concentration, it was precipitated in ether. After dissolving with absolute ethanol, it was repeatedly dissolved and precipitated in ether for 3 ti...
Embodiment 3
[0052] Embodiment 3: the synthesis of polyhydroxyethyl methacrylate-polymethyl methacrylate amphiphilic block polymer 3:
[0053] Dissolve 0.56g (0.1mmol) of initiator 2, 0.4g (4mmol) of methyl methacrylate and 0.0173g (0.1mmol) of pentamethyldiethylenetriamine in 2ml of dimethylformamide and place in a reaction flask; Nitrogen bubbling method was used to remove oxygen at room temperature for 30 minutes, and 0.0144 g (0.1 mmol) of cuprous bromide was added under the protection of nitrogen, and after nitrogen was passed for 15 minutes, the reaction bottle was sealed and reacted at room temperature for 6 hours. The reaction was terminated by exposing the reaction solution to air. Add 10ml of tetrahydrofuran to dilute the reaction solution, remove copper ions through a neutral aluminum oxide column, the eluent ratio is absolute ethanol / tetrahydrofuran = 1:30, concentrate, precipitate in ether, dissolve with absolute ethanol Repeated dissolution and precipitation in ether for 3 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com