Small-molecule fluorescent probe for rapidly identifying superoxide radicals, preparation method and application thereof
A fluorescent probe and superoxide technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, analytical materials, etc., can solve the problems of long detection time and inability to realize cell imaging, and achieve simple post-processing process and resistance to other molecules Strong interference ability and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of probe compound NS-O:
[0022]
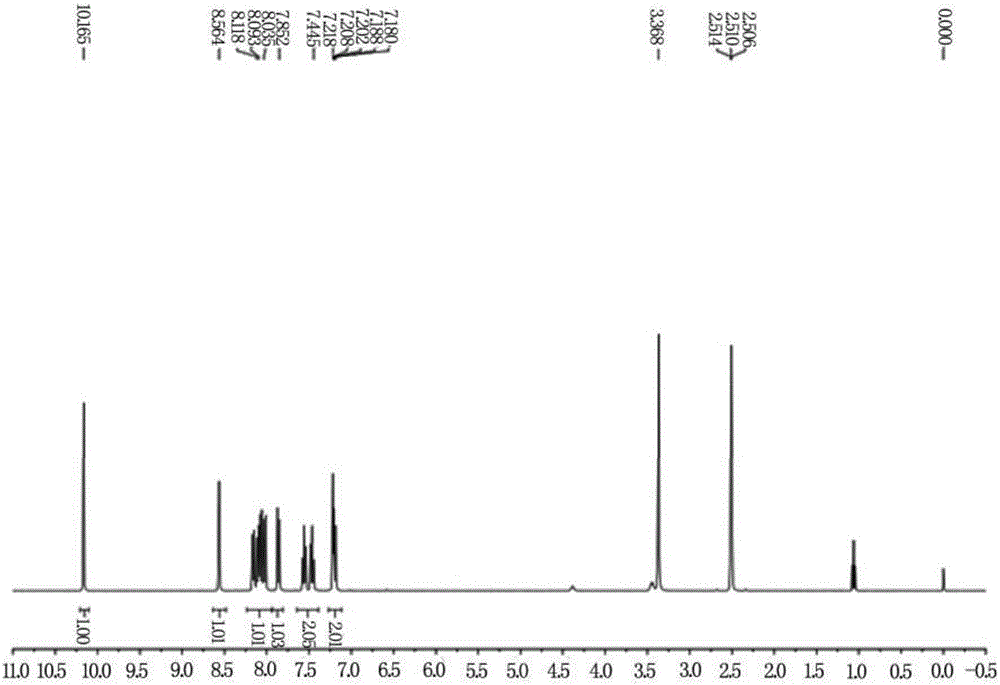
[0023] Compound 1 (0.5mmol, 86.0mg) and compound 2 (1.1equiv, 68.8mg) were added to the reaction flask under a nitrogen atmosphere, and then ethanol (5.0mL) was added to the above reactor at one time, and the After heating to reflux for 5 hours, the reaction was detected by spotting until the raw materials disappeared, and the solvent was spin-dried under reduced pressure to obtain a crude product, which was separated with a silica gel column to obtain the probe compound NS-O, the silica gel particle size was 200-300 mesh, and the yield was 63%. 1 H-NMR (400MHz, DMSO-d 6 )δ10.12(s,1H),8.56(s,1H),8.17-8.01(m,4H),7.86(d,J=8.8Hz,1H),7.58-7.45(m,2H),7.22-7.18 (m,2H). The probe's 1 H NMR spectrum see figure 1 .
Embodiment 2
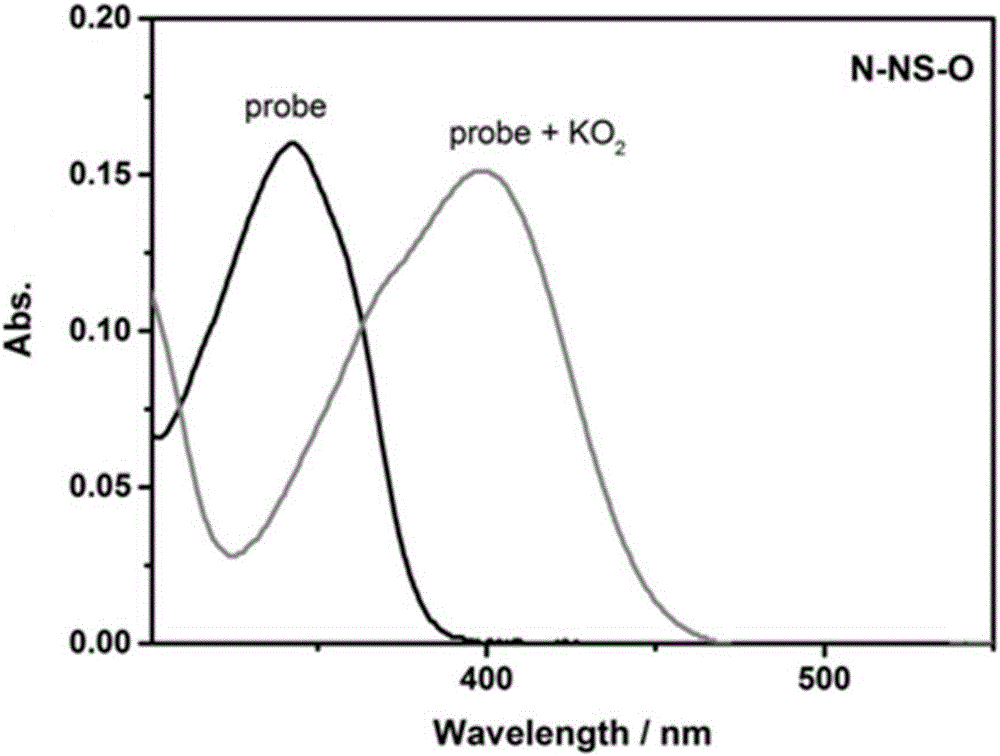
[0025] Probe NS-O with O 2 - The addition of its visible light absorption changes
[0026] The probe NS-O prepared in Example 1 was dissolved in N,N-dimethylformamide (DMF) to prepare a 1 mmol / L stock solution. Take 30 μL from the stock solution and add it to a 5mL centrifuge tube, measure its absorption spectrum, and then add O 2 - (10equiv) standard solution, diluted to 3mL (10μM) with DMSO / PBS (1:1, v / v), and measure its absorption spectrum. Spectrum see figure 2 shown. Depend on figure 2 It can be seen that after adding probe NS-O, directly add O 2 - , the change in absorption can be detected instantaneously, realizing the O 2 - detection.
Embodiment 3
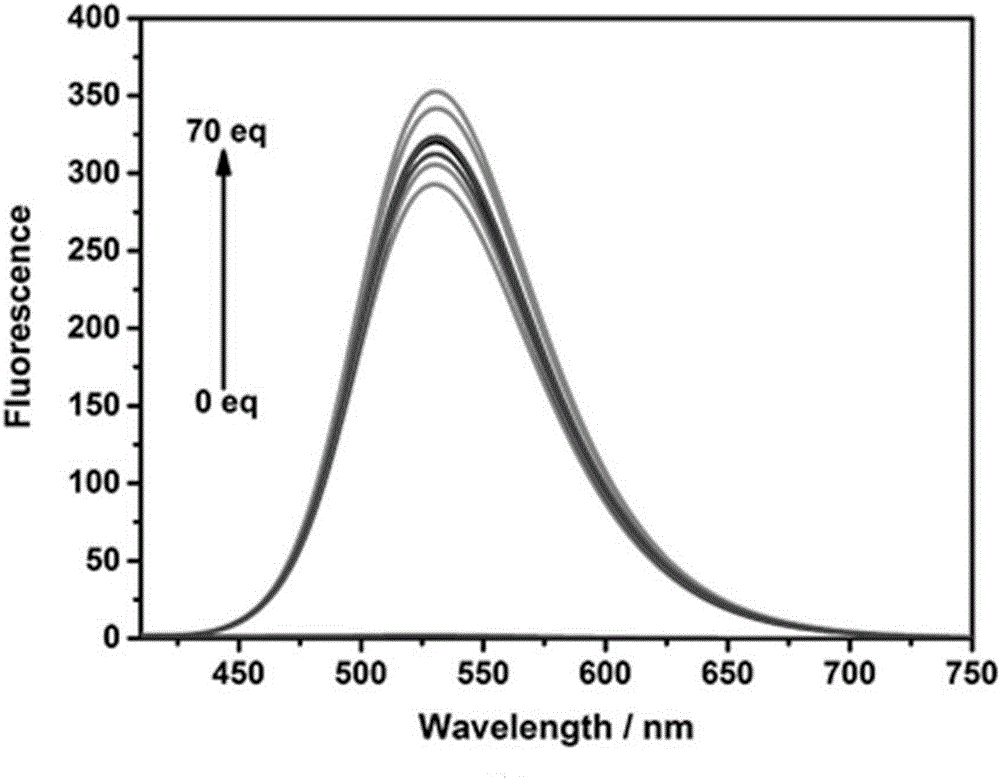
[0028] Probe compound NS-O fluorescent probe with O 2 - The change of fluorescence spectrum with the increase of equivalent
[0029] Take out 30 μ L and join among the centrifuge tube of 5mL from the stock solution of embodiment 2, add different equivalent (0-70equiv) O 2 - The standard solution was diluted to 3mL with PBS buffer solution (0.1mol / L, pH=7.4) and DMSO at a volume ratio of 1:1, and its fluorescence properties were measured with 400nm as excitation light. Fluorescence spectra such as image 3 shown. Depend on image 3 It can be seen that with O 2 - Fluorescence gradually increased with the addition of equivalents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com