Nitrosyl hydrogen molecular fluorescent probe for mitochondria targeting and preparation method and application thereof
A nitrosyl hydrogen and fluorescent probe technology, applied in the field of analytical chemistry, can solve problems such as DNA chain damage and body damage, and achieve the effects of good selectivity, easy synthesis, and strong resistance to other molecular interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
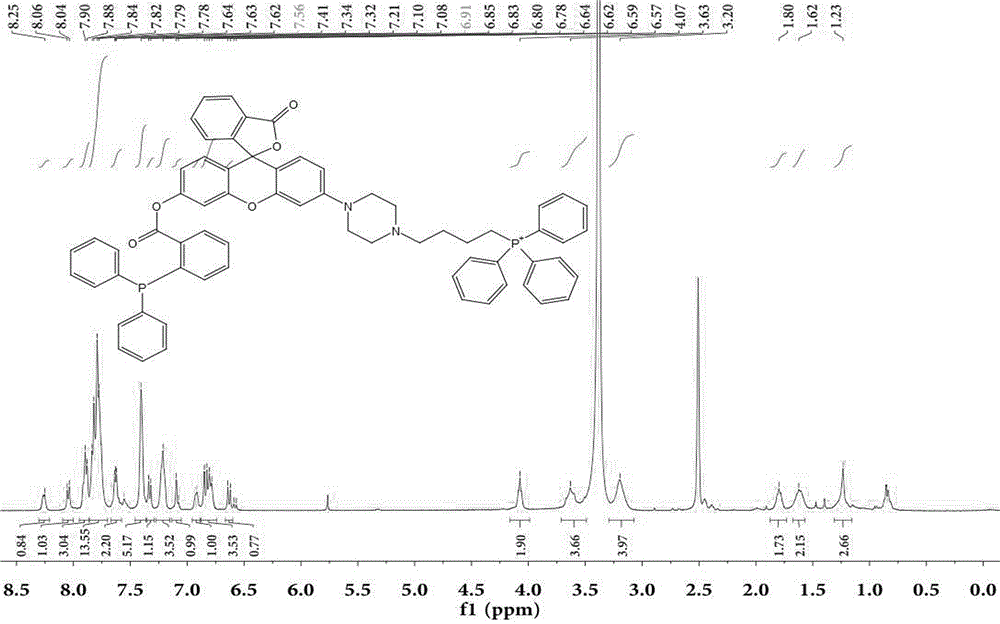
[0038] Synthesis of target probe Mito-HN
[0039] 1) Synthesis of compound MHN-1:
[0040]
[0041] The compound 2′,4′-dihydroxy-2-phenonebenzoic acid (1.0 g, 3.88 mmol, 1 eq) and m-hydroxyphenylpiperazine (758.3 mg, 4.26 mmol, 1.1 eq) were dissolved in 20 mL of trifluoroacetic acid, 90 o C was heated to reflux and reacted overnight. Use a TCL plate to detect the reaction. After the reaction is complete, spin dry the solvent trifluoroacetic acid under reduced pressure, dissolve it with 1ml ethyl acetate, and separate it with a silica gel column. The silica gel particle size is 200-300 mesh, and the eluent ratio is methanol / di Chloromethane=1:5. The yield was 81%.
[0042] 2) Synthesis of compound MHN-2:
[0043]
[0044] Compound MHN-2 (1.1g, 2.74mmol, 1eq), NaHCO 3(691mg, 8.22mmol, 3eq) was dissolved in 20mL acetonitrile and reacted at room temperature for 10min, then added Fmoc-Cl (850mg, 3.29mmol, 1.2eq), under nitrogen protection, reacted at room temperature fo...
Embodiment 2
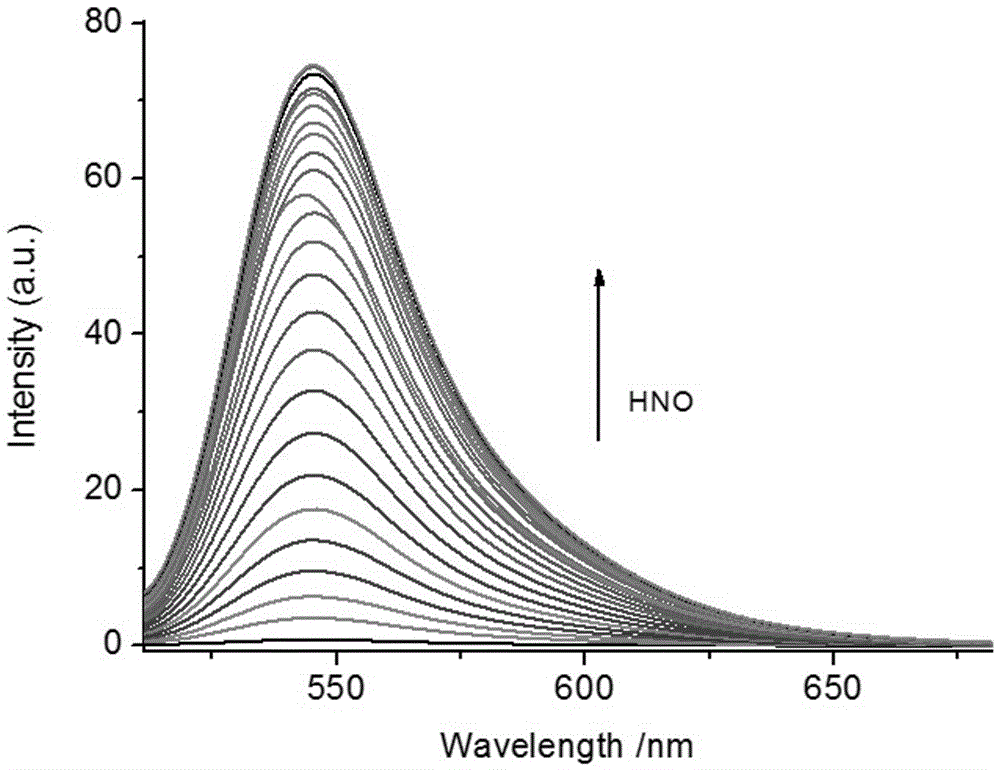
[0055] Changes of the fluorescence spectrum of the fluorescent probe Mito-HN with the increase of HNO addition
[0056] The Mito-HN fluorescent hydrogen nitrosyl probe prepared in Example 1 was dissolved in N,N-dimethylformamide (DMF) to prepare a 1 mmol / L stock solution. Take 30μL from the stock solution and add it to a 5mL centrifuge tube, dilute it with 2mL PBS (0.1mol / L, pH=7.4), then add different equivalents (0-10eq) of AS salt (HNO donor) standard solution, use The PBS buffer solution solution was diluted to 3mL, and its fluorescence properties were measured with 450nm as the excitation light. Fluorescence spectra such as figure 2 shown. Depend on figure 2 It can be seen that the fluorescence at 545nm increases gradually with the increase of the amount of HNO added.
Embodiment 3
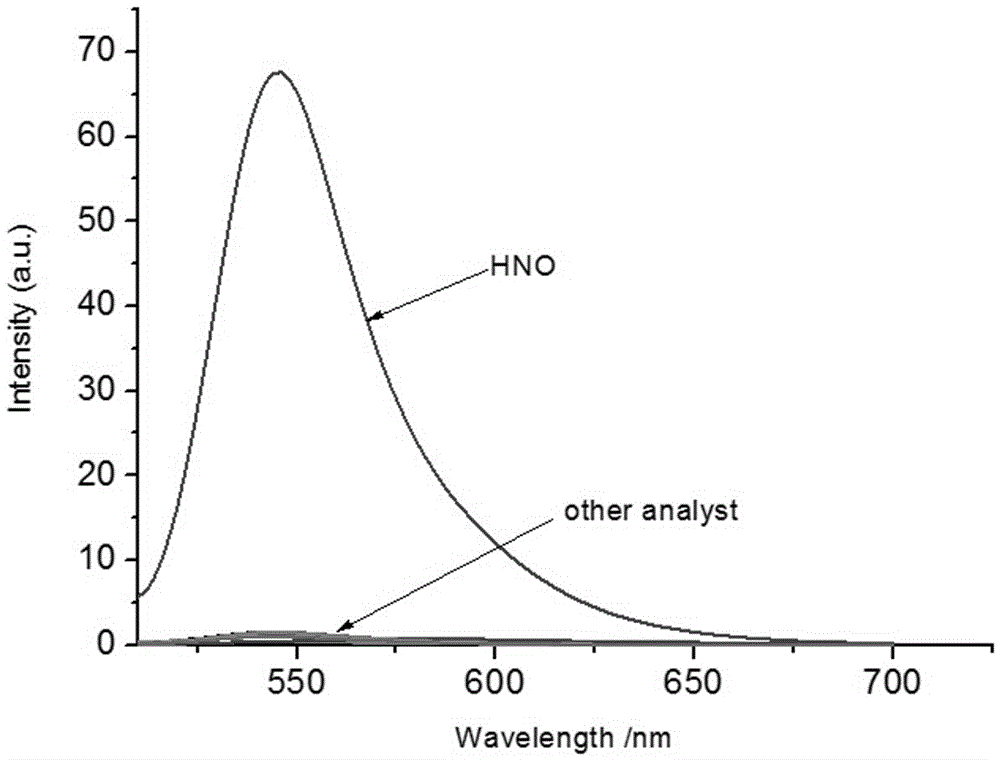
[0058] The selectivity of compound Mito-HN nitrosyl hydrogen fluorescent probe to different molecules or ions
[0059] Take 30 μL from the fluorescent probe stock solution in Example 2 and add them to 18 5mL centrifuge tubes respectively. After diluting with 2mL PBS, add equimolar amounts of competing molecular standard solutions to 16 centrifuge tubes respectively, one of which Do not add any ions, as a blank sample, and add an equimolar amount of HNO standard solution to the other, and detect the change of the fluorescence emission spectrum of the solution after 15 minutes, with 450nm as the excitation light, the results are as follows: image 3 with Figure 4 shown. Depend on image 3 with Figure 4 It can be found that other metal ions, reducing compounds, oxidizing compounds, etc. have almost no effect on the fluorescence of compound Mito-HN at 545nm, but the addition of nitrosyl hydrogen solution can significantly enhance the fluorescence of compound Mito-HN at 545nm....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com