Preparation method of substituted 1,2,3,4-tetrahydroquinoline
A technology for preparing tetrahydroquinoline and hydrogen, which is used in pharmaceutical and natural compound chemical intermediates and related chemical fields, can solve the problems of difficult separation and recovery, high price and high hydrogen pressure, and achieves simple operation and post-processing, and catalyst reproduction. The effect of good performance and high product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
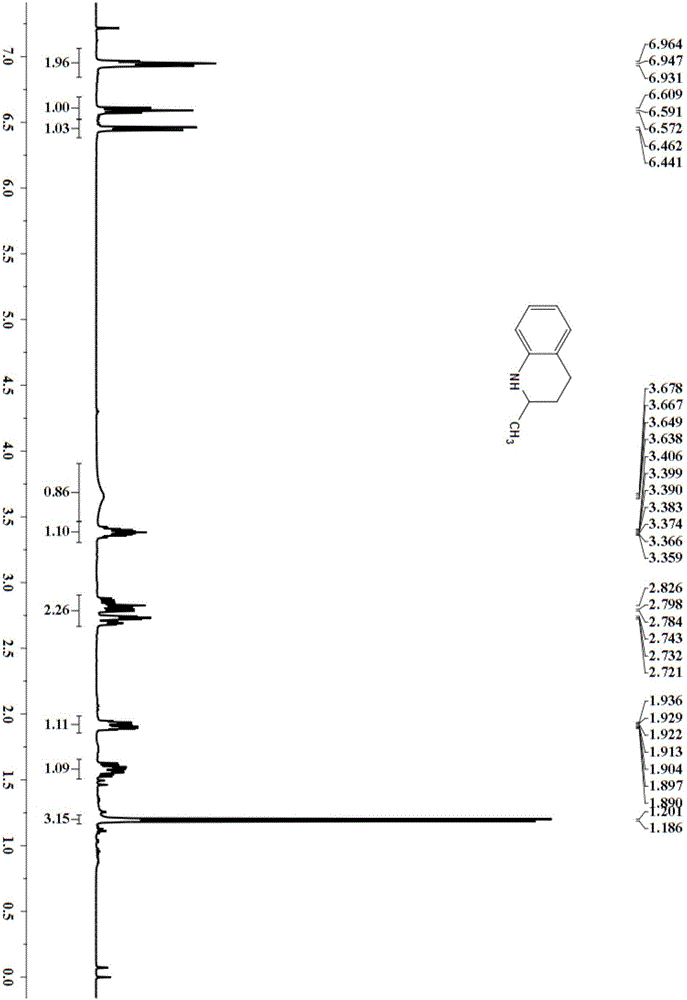
[0024] Embodiment 1: the synthesis of 2-methyl-1,2,3,4-tetrahydroquinoline
[0025] Add the substrate 2-methylquinoline (71.6mg, 0.5mmol), hydrogen (8bar) to the dioxane (5mL) solvent with PdNPore (2.7mg, 5mol%) catalyst, place in a magnetic stirrer Reaction at 80°C for 16 hours, column chromatography (silica gel, 200-300 mesh; developer, petroleum ether, ethyl acetate) to obtain 58.89 mg of 2-methyl-1,2,3,4-tetrahydroquinoline, the product Rate 80%.
[0026]
[0027] 2-Methyl-1,2,3,4-tetrahydroquinoline
[0028] 1 H NMR (400MHz, CDCl 3 )δ 6.98-6.92(m,2H),6.62-6.55(m,1H),6.45(d,J=8.4Hz,1H),3.66(br,1H),3.45-3.30(m,1H),2.89- 2.65(m,2H),1.95-1.87(m,1H),1.63-1.50(m,1H),1.19(d,J=6Hz,3H).
Embodiment 2
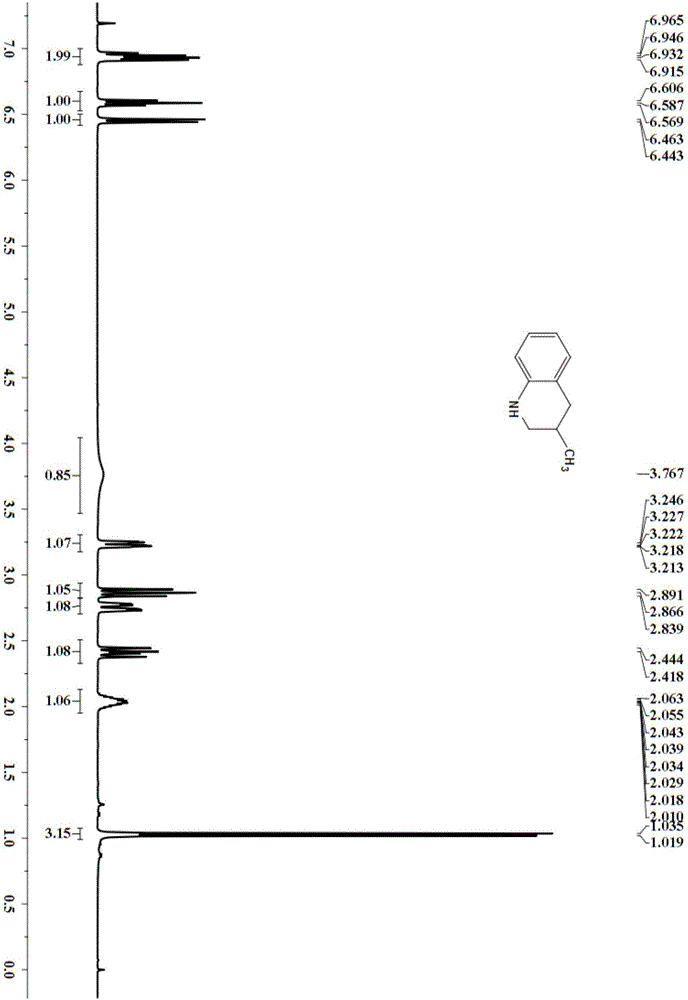
[0029] Embodiment 2: the synthesis of 2-methyl-1,2,3,4-tetrahydroquinoline
[0030] In the N,N-dimethylformamide (3mL) solvent that is added with PdNPore (5.4mg, 10mol%) catalyst, add substrate 2-methylquinoline (42.96mg, 0.3mmol), hydrogen (5bar), Put it on a magnetic stirrer and react at 30°C for 20h. Column chromatography (silica gel, 200-300 mesh; developer, petroleum ether, ethyl acetate) gives 2-methyl-1,2,3,4-tetrahydroquinone 30.92 mg of morphine, yield 70%.
[0031]
[0032] 2-Methyl-1,2,3,4-tetrahydroquinoline
[0033] 1 H NMR (400MHz, CDCl 3 )δ 6.98-6.92(m,2H),6.62-6.55(m,1H),6.45(d,J=8.4Hz,1H),3.66(br,1H),3.45-3.30(m,1H),2.89- 2.65(m,2H),1.95-1.87(m,1H),1.63-1.50(m,1H),1.19(d,J=6Hz,3H).
Embodiment 3
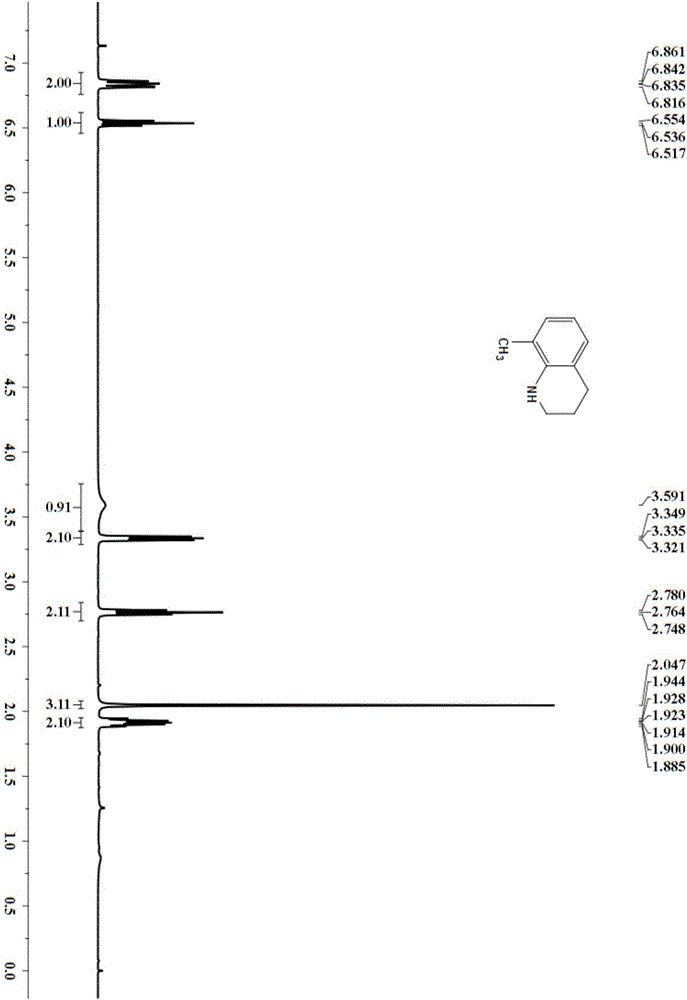
[0034] Embodiment 3: the synthesis of 3-methyl-1,2,3,4-tetrahydroquinoline
[0035] Add the substrate 3-methylquinoline (71.6mg, 0.5mmol) and hydrogen (3bar) to the ethanol (2mL) solvent with PdNPore (3.2mg, 6mol%) catalyst, and place it on a magnetic stirrer at 60°C After 12 hours of reaction, column chromatography (silica gel, 200-300 mesh; developer, petroleum ether, ethyl acetate) gave 47.85 mg of 3-methyl-1,2,3,4-tetrahydroquinoline, yield 65% .
[0036]
[0037] 3-Methyl-1,2,3,4-tetrahydroquinoline
[0038] 1 H NMR (CDCl 3,400MHz)δ:6.98-6.91(m,2H),6.59(t,J=7.2Hz,1H),6.45(d,J=8Hz,1H),3.77(br,1H),3.23-3.21(m, 1H), 2.87(t, J=10.8Hz, 2H), 2.79-2.73(m, 1H), 2.45-2.37(m, 1H), 2.08-2.0(m, 1H), 1.03(d, J=6.4Hz ,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com