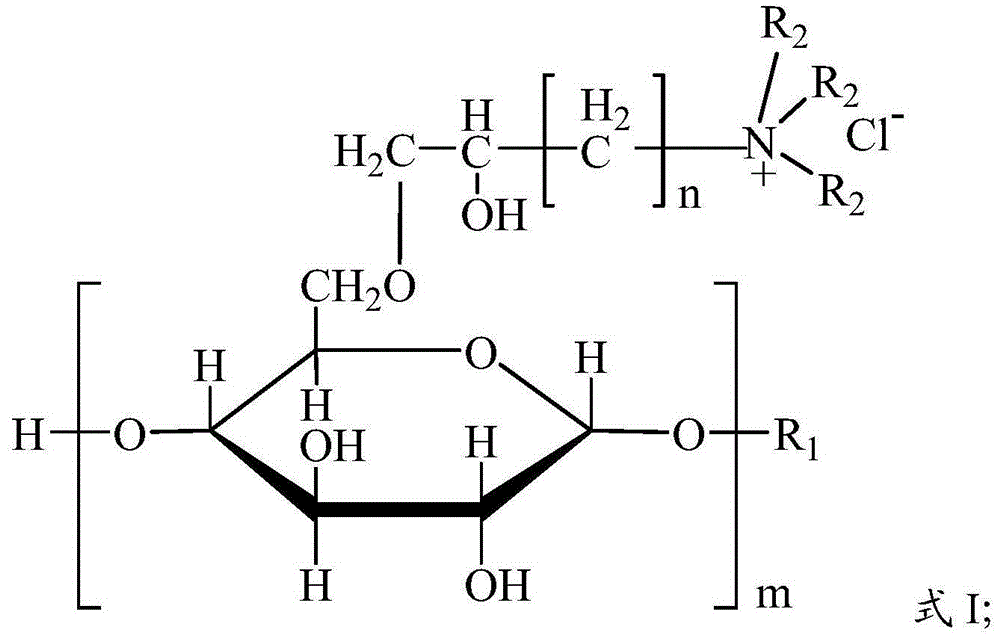
Glucoside, preparation method and applications thereof
A technology of alkyl glucoside and cation, applied in the field of cationic alkyl glucoside and its preparation, can solve the problems of cationic alkyl glucoside inhibition performance, poor lubricating performance, poor fluid loss reduction performance, limited wide application, etc., and achieves improved shear force. , The production process is simple, the effect of good inhibition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The present invention provides a kind of preparation method of cationic alkyl glucoside described in above-mentioned technical scheme, comprising:
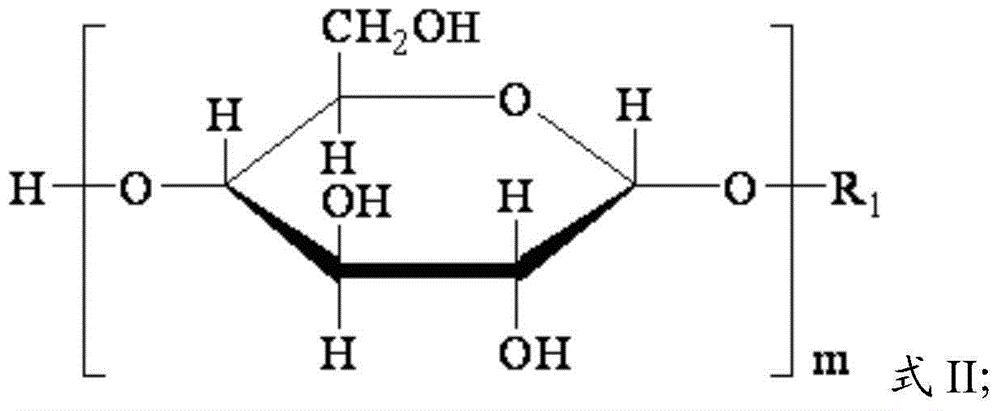
[0038] Alkyl glucoside, chlorinated epoxide, acidic catalyst and water are reacted to obtain intermediate product, and described alkyl glucoside has the structure shown in formula II:
[0039]
[0040] In formula II, R 1 is an alkyl group with 1 to 5 carbon atoms, and m is 1 to 3;
[0041] The chlorinated epoxides include epichlorohydrin or 1,2-epoxychlorobutane;
[0042] The intermediate product, basic compound and tertiary amine hydrochloride are reacted to obtain cationic alkyl glucoside, and the tertiary amine hydrochloride includes trimethylamine hydrochloride, triethylamine hydrochloride, tripropylamine hydrochloride or tributylamine hydrochloride.
[0043] In the present invention, alkyl glucoside, chlorinated epoxide, acid catalyst and water are reacted to obtain intermediate product. In an embodiment of the ...
Embodiment 1
[0070] Add 1000g of methyl glucoside, 500g of epichlorohydrin, 150g of hydrofluoric acid and 1500g of water into a large-capacity reactor equipped with a stirring, condensing and heating device, stir and mix evenly, react at 95°C for 2 hours, drop To room temperature, the intermediate product is obtained;
[0071] Add 160g of sodium hydroxide and 500g of trimethylamine hydrochloride to the above intermediate product, stir and mix evenly, react at 40°C for 0.5h, then add 10g of citric acid, dry the obtained reaction product to remove water, and obtain the cation Methyl glucoside, the yield is 96.23%.
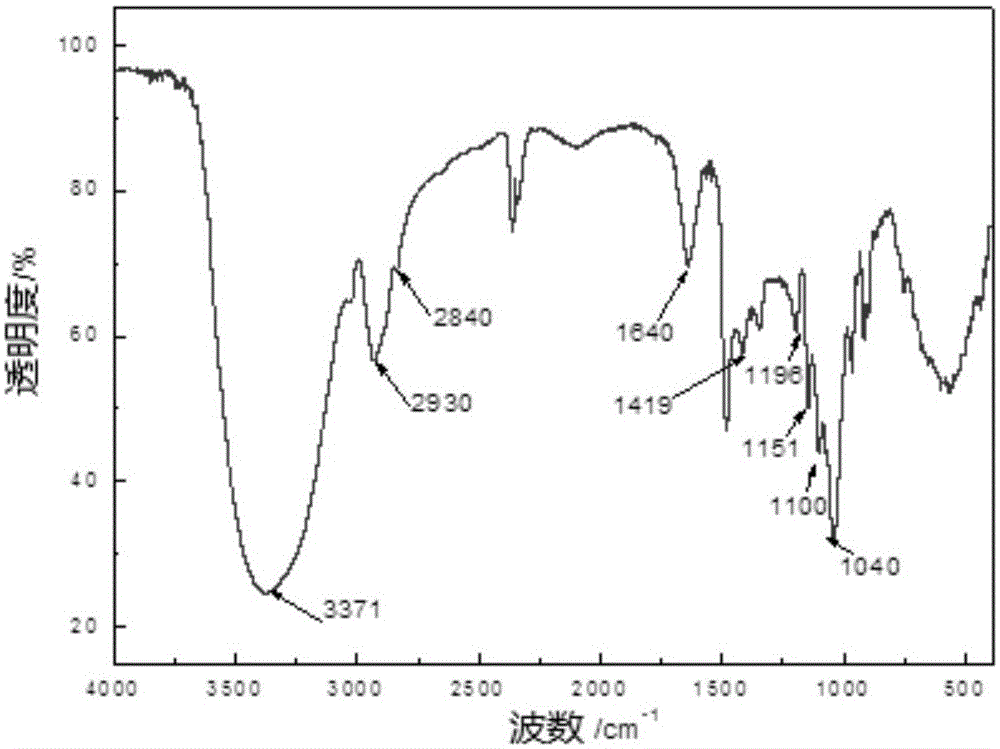
[0072] The cationic methyl glucoside prepared in Example 1 of the present invention is carried out infrared detection, and the detection result is as follows: figure 1 as shown, figure 1 For the infrared detection spectrum of the cationic methyl glucoside prepared in Example 1 of the present invention, by figure 1 It can be seen that the cationic methyl glucoside prepared in E...
Embodiment 2
[0077] Add 1100g of ethyl glucoside, 550g of 1,2-epoxychlorobutane, 170g of sulfuric acid and 1600g of water into a large-capacity reactor with stirring, condensation and heating devices, stir and mix evenly, and react at 100°C 3h, down to room temperature, the intermediate product is obtained;
[0078] Add 170g of sodium hydroxide and 550g of triethylamine hydrochloride to the above intermediate product, stir and mix evenly, react at 50°C for 1h, then add 11g of linalool, dry the obtained reaction product to obtain Cationic ethyl glucoside, the yield is 96.16%.
[0079] Infrared detection was carried out on the cationic ethyl glucoside prepared in Example 2 of the present invention, and the detection result was that the cationic ethyl glucoside prepared in Example 2 of the present invention had a structure shown in formula 2:
[0080]
[0081] In formula 2, m is 1~3, n is 2, R 1 for-C 2 h 5 , R 2 for-C 2 h 5 .
[0082] According to the method described in the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com