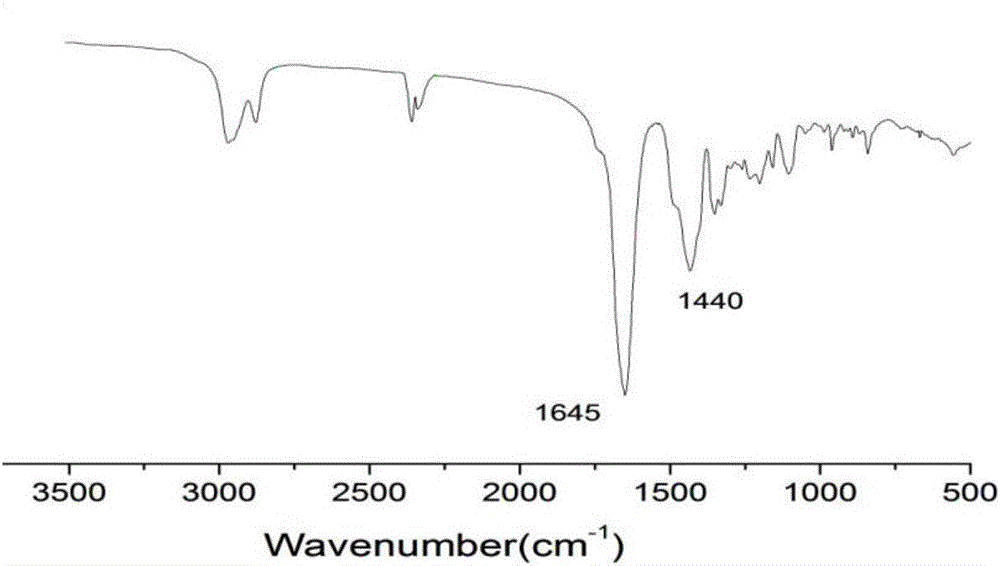
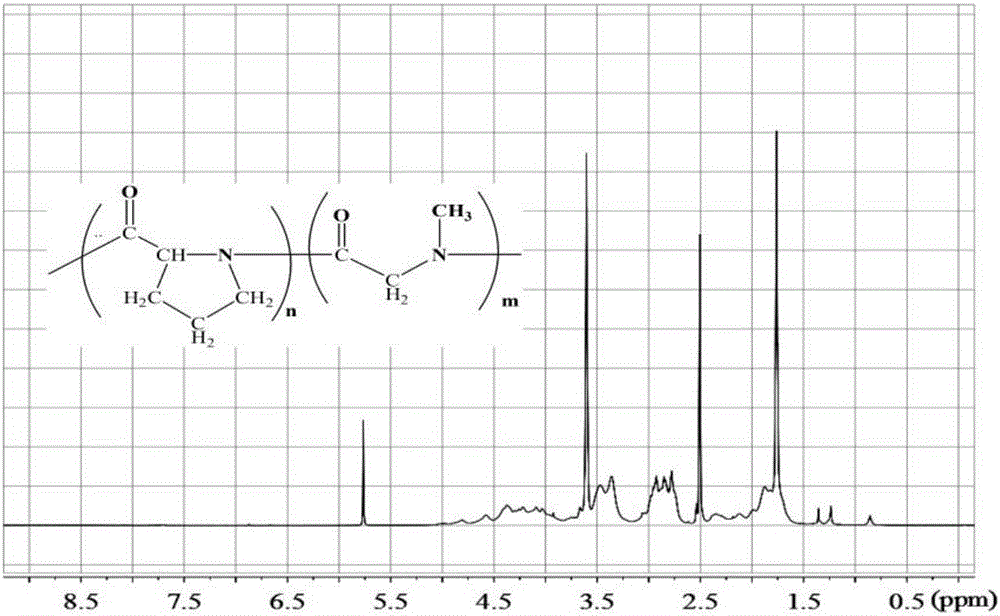
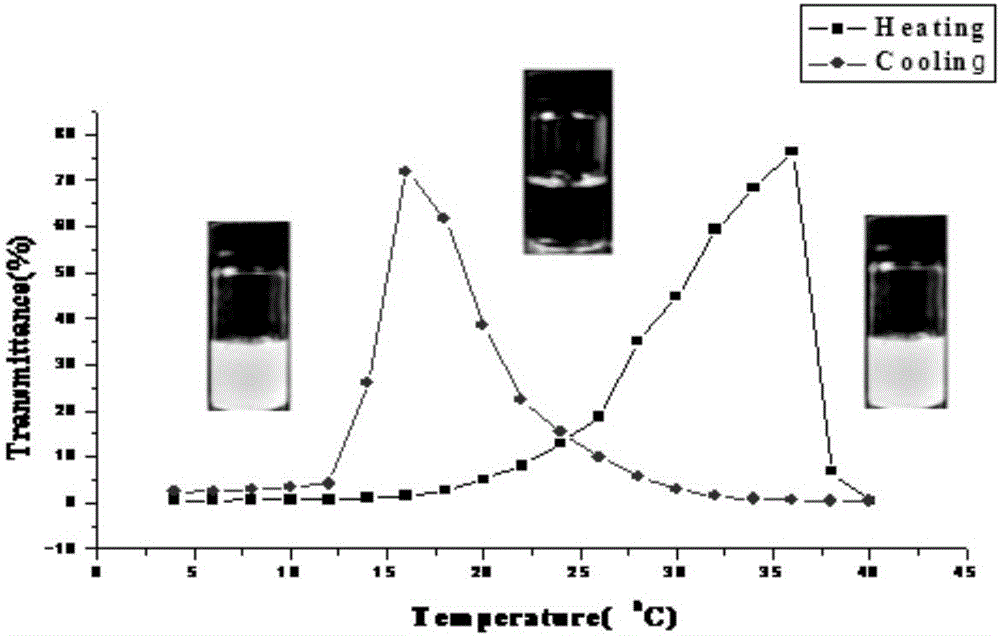
Thermo-sensitive poly(proline-creatine) material and preparation method thereof
A technology of proline and sarcosine, which is applied in the field of temperature-sensitive poly(proline-sarcosine) materials and their preparation, can solve the problem of not being able to have biocompatibility, biodegradability and temperature sensitivity at the same time , single and other problems, to achieve the effect of excellent biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of anhydride in N-carboxy-sarcosine ring
[0040] Add 2.0 g of sarcosine and 40 mL of anhydrous THF into a 100 mL three-necked round-bottomed flask equipped with a reflux condenser, and add 3.0 g of triphosgene in the oil bath to 60°C. React for about 2 hours, stop the reaction when the reaction suspension turns from light yellow to clear. Then pass N into the reaction system 2 To remove triphosgene and by-product HCl gas that did not participate in the reaction. After 2h, it was subjected to rotary evaporation, the reaction solution was concentrated to 2-3 mL, and slowly dropped into 50 mL of anhydrous n-hexane. Let the system stand at room temperature for 48 hours. It can be observed that solid crystals appear at the bottom of the container. Pour off the supernatant, add anhydrous tetrahydrofuran to dissolve the solid crystals, sink into n-hexane again, and repeat 2-3 times to obtain a white powder. solid.
[0041] The reaction equation is as follows...
Embodiment 2
[0052] (1) Synthesis of anhydride in N-carboxy-sarcosine ring
[0053] Add 3.0 g of sarcosine and 50 mL of anhydrous THF into a 100 mL three-neck round bottom flask equipped with a reflux condenser, and add 4.50 g of triphosgene in the oil bath to 50°C. After about 2.5 hours of reaction, stop the reaction when the reaction suspension turns from light yellow to clear. Then pass dry N into the reaction system 2 To remove triphosgene and by-product HCl gas that did not participate in the reaction. After 3 hours, it was subjected to rotary evaporation, the reaction solution was concentrated to about 6 mL, and slowly dropped into 50 mL of anhydrous petroleum ether / n-hexane mixed solvent. The system was left standing at room temperature for 36 hours, and solid crystallization could be observed at the bottom of the container. The supernatant was removed by filtration, and anhydrous tetrahydrofuran was added to dissolve the solid, which was then sunk into n-hexane again. This opera...
Embodiment 3
[0065] (1) Synthesis of anhydride in N-carboxy-sarcosine ring
[0066] Add 2.0 g of sarcosine and 40 mL of anhydrous chloroform into a 100 mL three-necked round-bottom flask, and heat the oil bath to 50° C., then add 3.0 g of triphosgene. After 3.5 hours of reaction, the reaction system changed from a suspension to a clear state, and the reaction was stopped. Pass N into the reaction system 2 To remove triphosgene and by-product HCl gas that did not participate in the reaction. After 2.5 h, it was subjected to rotary evaporation, the reaction liquid was concentrated to about 4 mL, and slowly dropped into 40 mL of anhydrous n-hexane. The system was left standing at room temperature for 50 h, and solid crystallization could be observed at the bottom of the container. Pour out the supernatant, add anhydrous tetrahydrofuran to dissolve the solid crystals, sink into n-hexane again, and repeat this operation 3-4 times to obtain N-carboxy-sarcosine cyclic anhydride. The reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com