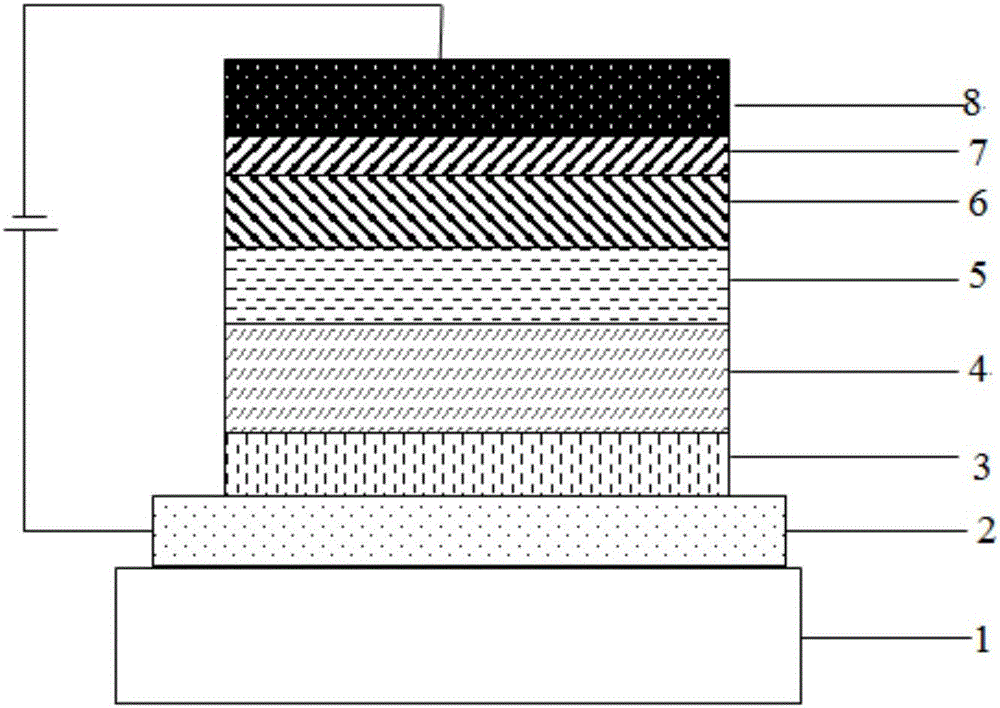
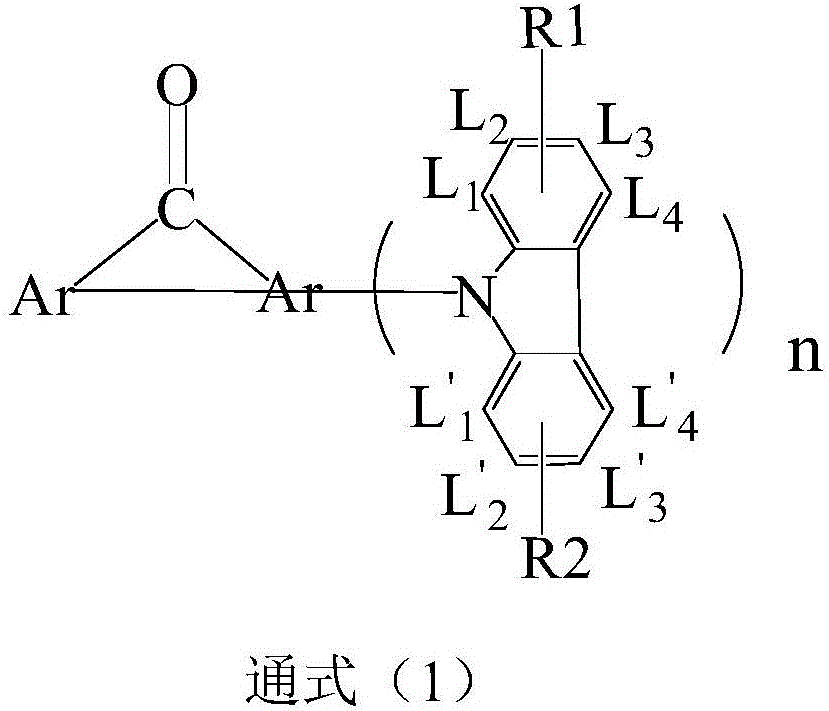
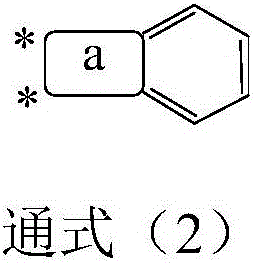
Compound based on diaryl ketone and application thereof
A diaryl ketone and compound technology, applied in the field of semiconductors, can solve the problems of efficiency roll-off, low S1 state radiation transition rate, difficult exciton utilization rate and high fluorescence radiation efficiency, etc., to achieve device life improvement and good film formation Sex and fluorescence quantum efficiency, the effect of avoiding aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the synthesis of compound 3
[0063] synthetic route:
[0064]
[0065] In a 250ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 4,4'-dibromobenzophenone, 0.025mol intermediate A, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, the reaction was complete; naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 98.9% and a yield of 65%.
[0066] Elemental analysis structure (molecular formula C 53 h 32 N 2 O): Theoretical value C, 89.30; H, 4.52; N, 3.93; O, 2.24; Test value: C, 89.31; H, 4.51; N, 3.92;
[0067] HPLC-MS: The molecular weight of the material is 712.83, and the measured molecular weight is 713.05.
Embodiment 2
[0068] Embodiment 2: the synthesis of compound 10
[0069] synthetic route:
[0070]
[0071] In a 250ml four-neck flask, under nitrogen atmosphere, add 0.01mol 4-bromobenzophenone, 0.015mol intermediate B, 0.02mol sodium tert-butoxide, 5×10 -5 mol Pd 2 (dba) 3 , 5×10 -5 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampled and plated, the reaction was complete; naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 99.4% and a yield of 80%.
[0072] Elemental analysis structure (molecular formula C 37 h 24 N 2 O): theoretical value C, 86.69; H, 4.72; N, 5.46; O, 3.12; tested value: C, 86.69; H, 4.73; N, 5.47;
[0073] HPLC-MS: The molecular weight of the material is 512.60, and the measured molecular weight is 512.85.
Embodiment 3
[0074] Embodiment 3: the synthesis of compound 15
[0075] synthetic route:
[0076]
[0077] In a 250ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 4,4'-dibromobenzophenone, 0.025mol intermediate C, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, the reaction was complete; naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 99.1% and a yield of 65%.
[0078] Elemental analysis structure (molecular formula C 49 h 28 N 2 o 3 ): theoretical value C, 84.95; H, 4.07; N, 4.04; O, 6.93; test value: C, 84.94; H, 4.08; N, 4.03;
[0079] HPLC-MS: The molecular weight of the material is 692.76, and the measured molecular weight is 692.97.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com