Injectable bone substitutes for augmenting implant fixation
A technology for implants, uses, applications, bone diseases, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] Preparation of the bone cavity may require removal of damaged bone, and in the case of removal of a pre-existing prosthesis, this may include removal of the PMMA cement to provide a bone cavity suitable to receive and secure the prosthesis. Suitably, a straight box or offset chisel may be used to orient the bone canal into which the prosthesis is to be implanted, and the canal cleared to accept a starter reamer. Opening into the distal portion of the bone canal can then be initiated using a single priming reamer on the T-handle, where the reamer is introduced to a level appropriate for preoperative X-ray templated prosthesis dimensions. The proximal portion of the bone canal can then be enlarged using one or more upsizing broaches until a template implant size is reached, which is selected to ensure a tight fit of the prosthesis to be implanted. After the bone substitute composition has been introduced into the cavity, the prosthetic stem can be advanced into place by a...
Embodiment 1
[0102] Example 1 Preparation of injectable biphasic ceramic bone substitute composition
[0103] In this example, three different types of hardenable ceramic bone substitute materials were prepared. All three samples consisted of 59.6wt% α-CSH, 40.0wt% HA, 0.4wt% CSD, and had the same liquid-to-powder ratio (L / P=0.43mL / g), but the liquid phase and the added There are different types of compounds, see the table below.
[0104] sample name liquid phase Added compound CSH / HA Iohexol (180mg I / mL) - CSH / HA+gentamycin brine Gentamicin Sulfate CSH / HA+Vancomycin Iohexol (180mg I / mL) Vancomycin Hydrochloride
[0105] CSH / HA
[0106] Mixing 11.6 g of ceramic bone substitute with 5.0 mL of a liquid phase containing iohexol (180 mg I / mL) resulted in an L / P ratio of 0.43 mL / g. Mix for 30 seconds using a specially designed mixing and injection device (WO 2005 / 122971). The resulting paste can be injected with a 16G needle for up to 5 minutes an...
Embodiment 2
[0114] Example 2 Demonstration of Injectable Biphasic Ceramic Bone Substitute Compositions Comprising Vancomycin Enhances Implant Fixation Using a Model System
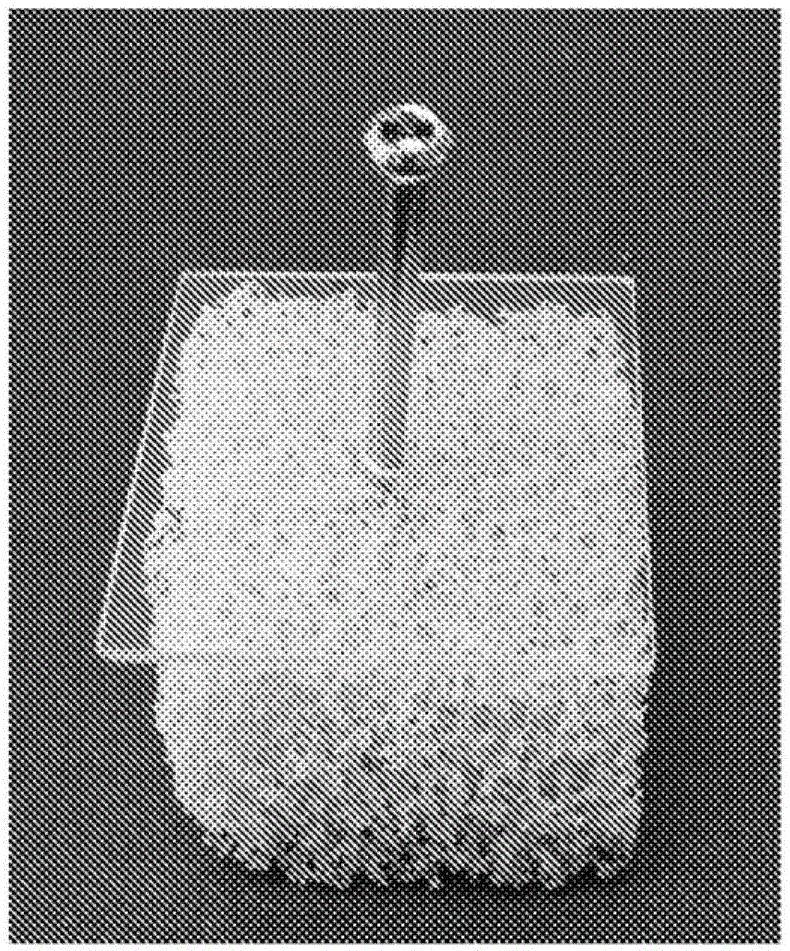
[0115] The effect of the injectable composition prepared according to Example 1 on implant fixation was determined in a model system by determining the pull-out force and torsion resistance of screws inserted into cancellous bone models that had been augmented with the injectable composition.
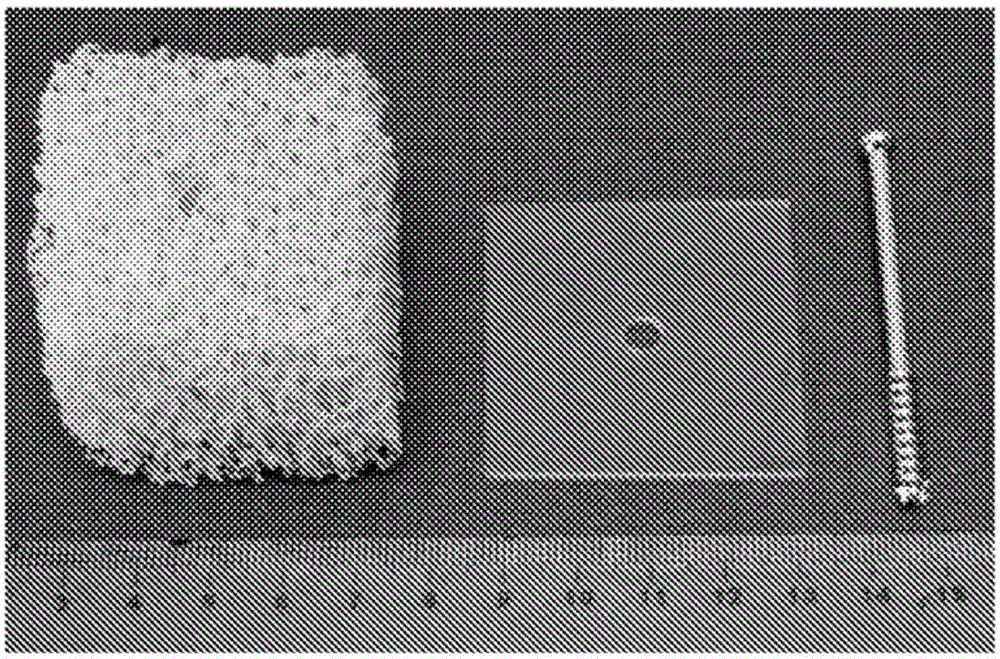
[0116] The spongy bone model consists of (Sawbones.com) rigid open cell foam block (Product No. 1522-507). The foam block has a more than 95% open pore structure with an open cell size of 1.5-2.5 mm, similar to human cancellous bone, making it suitable for dynamic testing or cement injection. The foam block has a density of 0.12g / cc, a compressive strength of 0.28MPa, and a compressive modulus of 18.6MPa, which are relatively low in the order and are used because they most closely simulate osteoporotic bone, where the fixatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
| compressive modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com