A swine pseudorabies virus variant XF-1 strain, a preparing method thereof and applications of the strain
A technology for porcine pseudorabies and virus mutation, applied in biochemical equipment and methods, viruses, antiviral agents, etc., can solve problems such as differences in immunogenicity, achieve high antibody levels, reduce labor costs, and reduce the effects of stress response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of porcine pseudorabies virus (XF-1 strain) liquid and vaccine:
[0037] 1. Suspension culture of BHK-21 cells in a bioreactor:
[0038] Take 5ml of BHK-21 seed cells preserved in liquid nitrogen, melt rapidly in a 37°C water bath, centrifuge at 1000rpm for 5 minutes, discard the supernatant, resuspend with 100ml of BHK medium containing 2% (v / v) low serum, and transfer to In a 250ml shake flask, culture on a shaker at a constant temperature at 37°C with a rotation speed of 80-100rpm / min for suspension culture for 2 days until the cell density reaches 2.0-4.0×10 6 cells / ml, according to the ratio of 1:4, at 37 ° C, 80 ~ 100 rpm for 2 ~ 3 days, the cells were expanded to a volume of 1000 ml, so that the cell density reached 4.0 ~ 6.0 × 10 6 cells / ml in order to obtain BHK-21 cell suspension.
[0039] Inoculate 1000ml of the BHK-21 cell suspension obtained above into a 10L bioreactor, add 8L of BHK medium containing 2% (v / v) newborn bovine serum, control the...
Embodiment 2
[0047] Immunogenicity Test of Porcine Pseudorabies Inactivated Vaccine (XF-1 Strain)
[0048] 1. Materials and methods
[0049] 1.1 The experimental animal market purchased 21-25-day-old piglets from a pig farm in Wuhan, and tested the main pathogens and related antibodies. A total of 30 piglets with negative neutralizing antibody against pseudorabies (PRV neutralizing antibody titer not higher than 1:2).
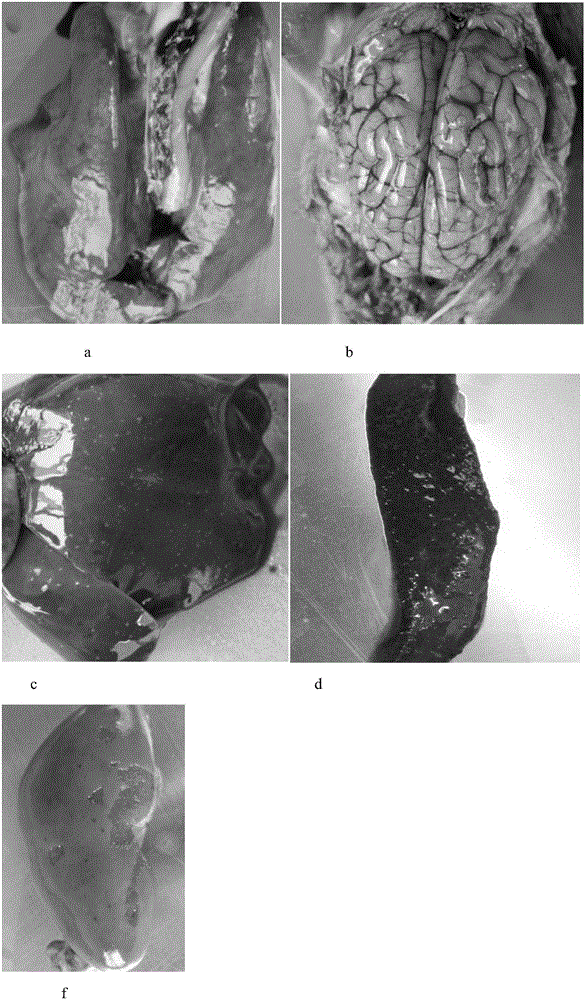
[0050] 1.2 The challenge strain The field strain of porcine pseudorabies virus (XF strain) comes from Wuhan Keqian Biotechnology Co., Ltd.
[0051] 1.3 Method The test pigs were randomly divided into 6 groups, 5 heads in each group, and the conditions of each group were as follows:
[0052] The 1st group is porcine pseudorabies inactivated vaccine (XF-1 strain), promptly selects the vaccine that embodiment 1 prepares;
[0053] The second group is the imported live vaccine (Bartha-K61 strain), batch number: 77BN, which was purchased from Spain's Hyperion Biopharmaceutical...
Embodiment 3
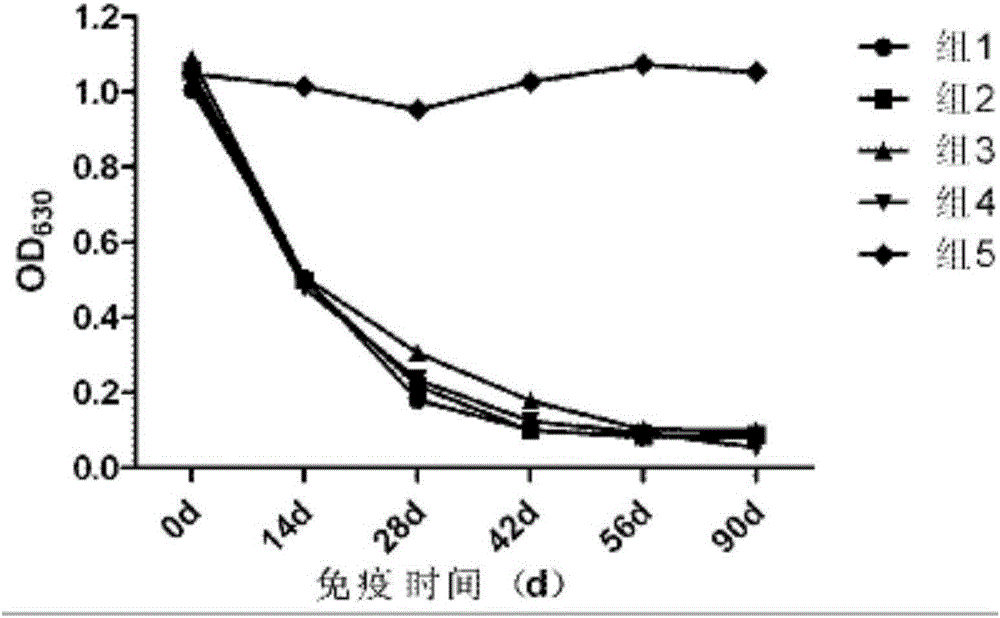
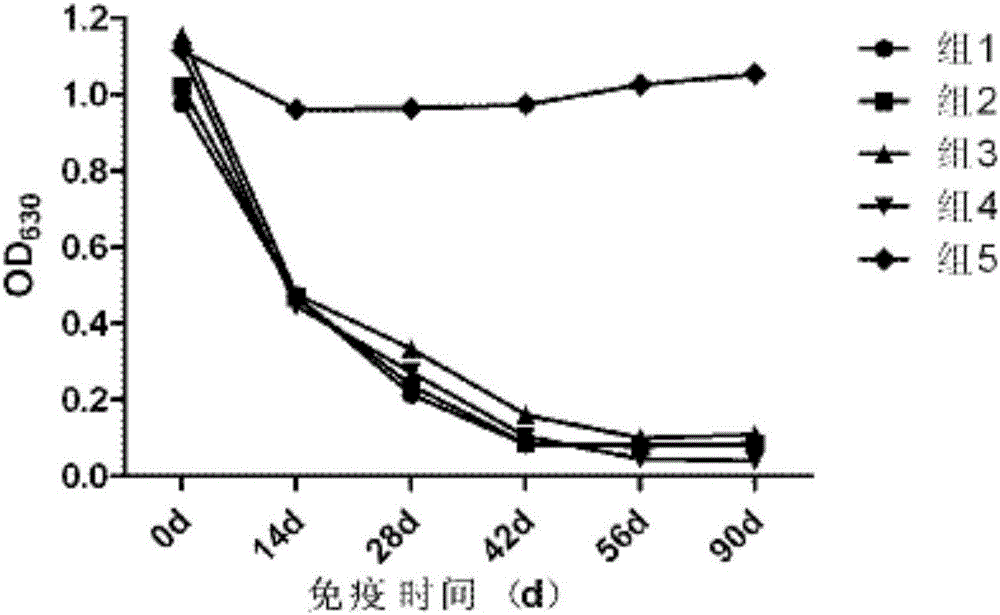
[0075] Comparative experiment on the growth and decline of antibodies against several different porcine pseudorabies vaccines
[0076] 1. Test materials and methods
[0077] 1.1 The experimental animal market purchased 21-25-day-old piglets from a pig farm in Wuhan, and tested the main pathogens and related antibodies. A total of 25 piglets with negative neutralizing antibody against pseudorabies (PRV neutralizing antibody titer not higher than 1:2).
[0078] 1.2 The neutralizing titer of porcine pseudorabies-specific positive serum is 1:256, provided by Wuhan Keqian Biotechnology Co., Ltd.
[0079] 1.3 Test method The test pigs were randomly divided into 5 groups, 5 heads in each group, and the grouping conditions were as follows:
[0080] The first group selects the vaccine prepared in Example 1 for porcine pseudorabies inactivated vaccine (XF-1 strain);
[0081]The second group is the imported live vaccine (Bartha-K61 strain), batch number: 77BN, purchased from the Spani...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Poison price | aaaaa | aaaaa |
| Poison price | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com