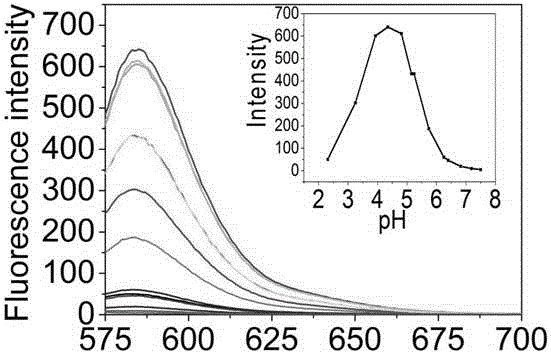
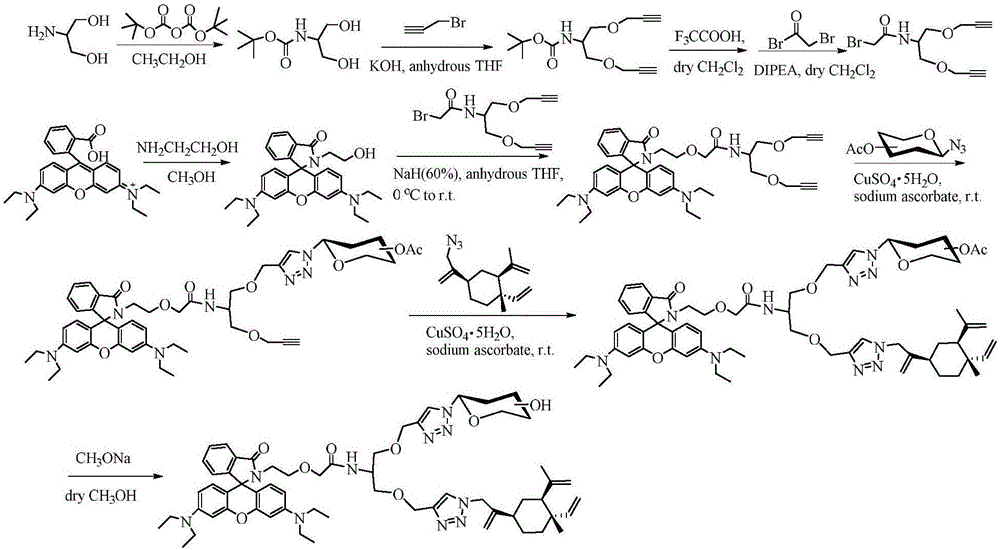
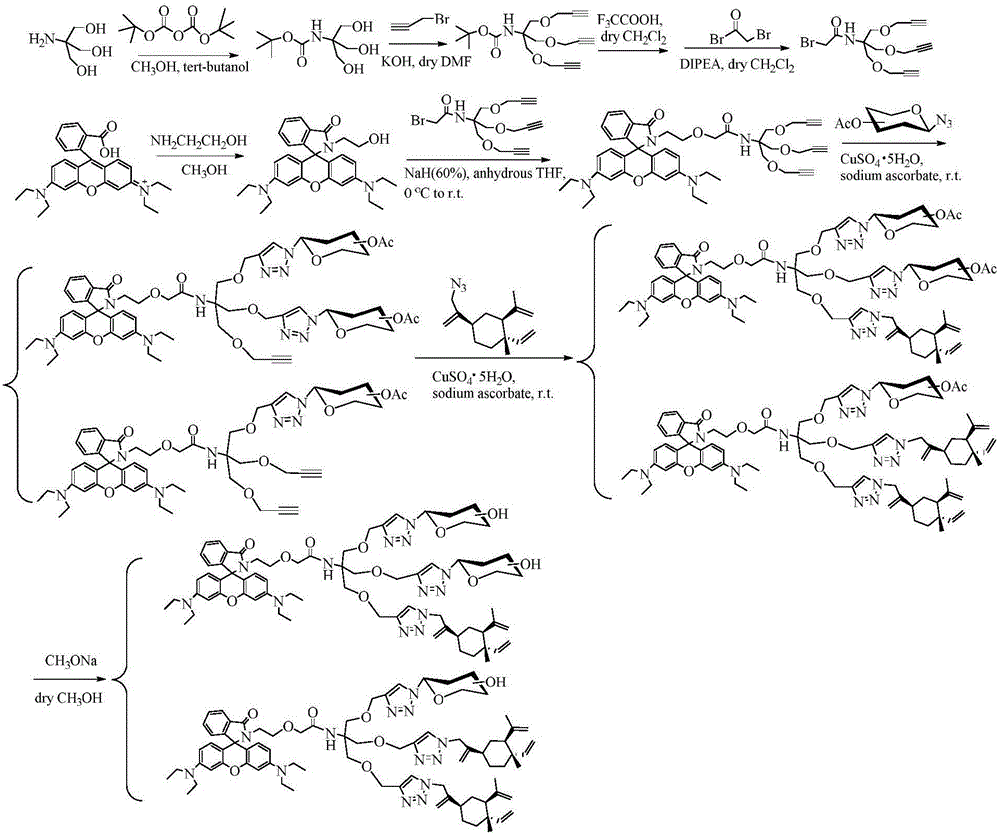
Glycosyl beta-elemene derivatives, preparation method and application thereof
A technology for elemene and derivatives, applied in the field of medicine, can solve the problems of inability to monitor treatment in real time, difficulty in β-elemene, and limited clinical application, and achieve long excitation wavelength, high quantum yield, and good water solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Dissolve rhodamine B (10g) in methanol (20mL) and add ethanolamine (25mL), protect with Ar, reflux in an oil bath at 75°C until the red color disappears, cool to room temperature, add 100mL deionized water, CH 2 Cl 2 Extraction, washed with saturated brine, anhydrous Na 2 SO 4 It was dried, filtered, concentrated, and purified by silica gel column (PE / EA=4:1) to obtain off-white solid 1 (3.84 g, 35%). 1 H NMR (600MHz, CDCl 3 )δ7.92(dd, J=5.6, 3.0Hz, 1H), 7.46(dd, J=5.6, 3.1Hz, 2H), 7.09(dd, J=5.2, 3.2Hz, 1H), 6.51(d, J =8.9Hz, 2H), 6.40(d, J=2.4Hz, 2H), 6.31(dd, J=8.9, 2.4Hz, 2H), 3.51-3.47(m, 2H), 3.36(q, J=7.0Hz , 8H), 3.32-3.29(m, 2H), 1.19(t, J=7.1Hz, 12H). 13 C NMR (151MHz, CDCl 3 )δ 170.10, 153.92, 153.28, 148.90, 132.69, 130.45, 128.51, 128.14, 123.81, 122.90, 108.24, 104.80, 97.80, 65.87, 62.68, 62.66, 44.65, 44.37, 12.
[0036]
[0037] Dissolve serinol (5 g) in 150 mL of absolute ethanol, add 100 mL of BOC anhydride (12 g) in absolute eth...
Embodiment 2
[0056]
[0057] Compound 12 was obtained by a synthetic method similar to compound 6. 1H NMR (600MHz, CDCl 3 )δ7.92-7.89 (m, 1H), 7.87 (s, 1H), 7.48-7.43 (m, 2H), 7.16-7.08 (m, 2H), 6.45 (ddd, J=8.6, 5.9, 2.6Hz, 2H), 6.39(t, J=2.6Hz, 2H), 6.29(ddd, J=8.2, 5.5, 2.6Hz, 2H), 5.86(dd, J=9.3, 2.7Hz, 1H), 5.49(q, J =9.4Hz, 1H), 5.44-5.40(m, 1H), 5.38(d, J=2.8Hz, 1H), 5.15(dd, J=10.4, 7.9Hz, 1H), 4.99(dd, J=10.4, 3.5Hz, 1H), 4.65(d, J=2.9Hz, 2H), 4.55(dd, J=7.9, 2.1Hz, 1H), 4.49(d, J=12.2Hz, 1H), 4.32-4.27(m, 1H), 4.18-4.12(m, 3H), 4.06-4.03(m, 2H), 4.03-3.98(m, 1H), 3.93(dt, J=10.5, 6.1Hz, 2H), 3.69(dd, J= 14.8, 3.5Hz, 1H), 3.65-3.61(m, 3H), 3.61-3.57(m, 2H), 3.48(dd, J=14.0, 6.9Hz, 1H), 3.39-3.32(m, 10H), 3.11 (dt, J=9.4, 6.0Hz, 1H), 3.03-2.98(m, 1H), 2.41(dd, J=2.3, 1.7Hz, 1H), 2.18(s, 3H), 2.11(s, 3H), 2.09(d, J=0.6Hz, 3H), 2.08(s, 3H), 2.07(s, 3H), 1.99(s, 3H), 1.87(d, J=4.5Hz, 3H), 1.18(td, J =7.0, 1.3Hz, 12H).
[0058]
[0059] Compound 13 was obtained by a synthe...
Embodiment 3
[0063]
[0064] Dissolve 5g of trishydroxymethylaminomethane in a mixed solution composed of 30mL of methanol and 30mL of tert-butanol, add BOC anhydride (11.75g) tert-butanol (50mL) solution under stirring, react overnight at room temperature, spin off the solvent, add ethyl acetate The ester was refrigerated overnight and filtered to give 15 (8.5 g, 93%) as a white solid. 1 H NMR (600MHz, DMSO-d6) δ4.49(s, 3H), 3.52(s, 6H), 1.37(s, 9H).
[0065]
[0066] A suspension of powdered KOH (2.3 g) and dry DMF (10 mL) was cooled to 0° C. under Ar protection. After compound 15 (1.5g) was dissolved in anhydrous THF (10mL), it was added dropwise to the reaction solution. After the addition was completed, it was reacted at 0°C for 10min, and 3.3mL of propyne bromide was added dropwise, and the temperature was raised to 35°C after 5min at 0°C. ℃ react overnight, add deionized water (100mL), CH 2 Cl 2 Extraction, washed with saturated brine, anhydrous Na 2 SO 4 It was dried, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com