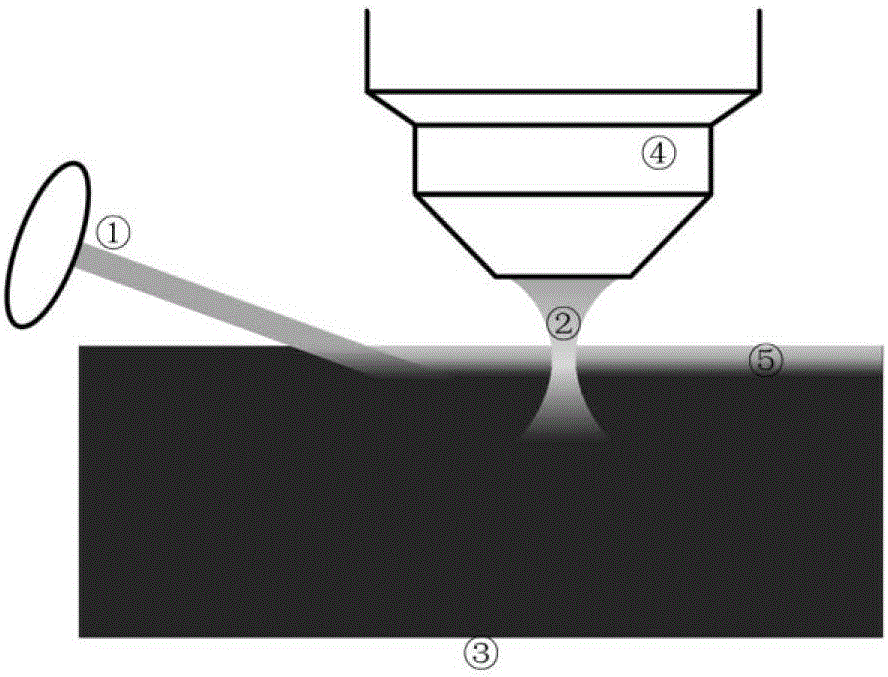
Fluorescence control method of light-controlled fluorescent protein marker biological tissue embedding sample
A fluorescent protein and control method technology, which is applied in the field of fluorescence control of light-controlled fluorescent protein-labeled biological tissue-embedded samples, can solve the problems of slow imaging of biological tissue-embedded samples, poor tomographic ability, and low axial resolution. Achieve the effect of convenient activation, precise control and good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
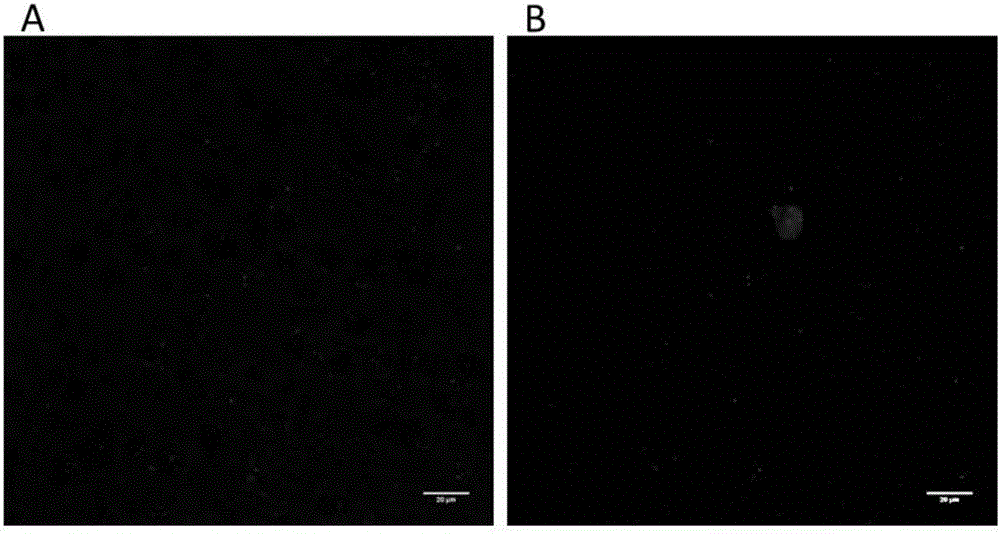
[0051] mEos3.1 is a common photoconvertible fluorescent protein. Its optical properties are that it only emits green fluorescence (excitation wavelength 488nm) and does not emit red fluorescence before being activated by ultraviolet 405nm wavelength; after being activated by ultraviolet 405nm wavelength for a short time, it emits red fluorescence under 561nm excitation light (emission spectrum is in above 550nm).
[0052] The method for embedding and light-sheet fluorescence control and fluorescence tomography of the mouse whole brain injected with Rv-dg-mEos3.1 in the cerebral cortex comprises the following steps:
[0053] (1) Sample fixation
[0054] The whole brain of the Rv-dg-mEos3.1-infected mouse was fixed by means of chemical fixation to obtain fixed mEos3.1-labeled biological tissue. Specific steps are as follows:
[0055] After the heart was perfused at 4°C, the dissected whole brain of the mouse was soaked in 4% PFA solution for about 12 hours. Use 40ml of PBS s...
Embodiment 2
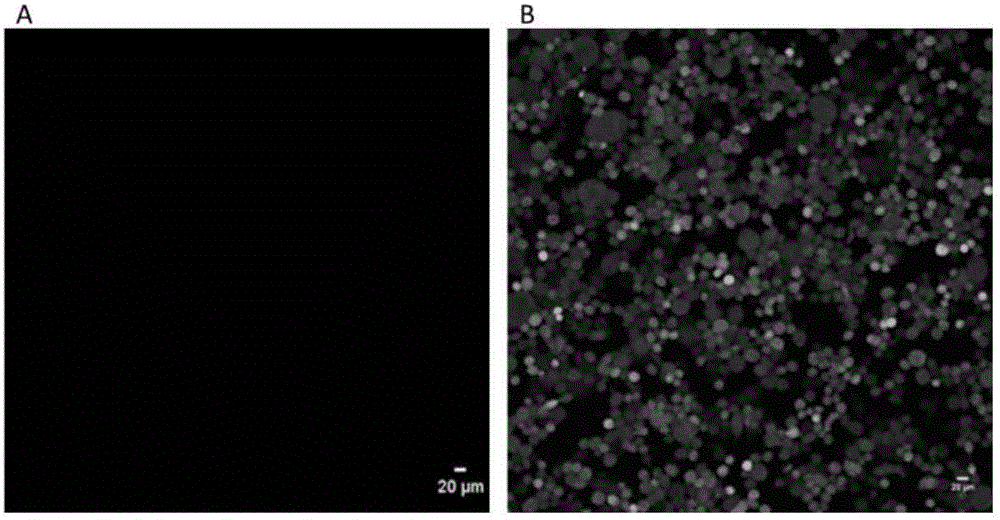
[0069] The method for controlling fluorescence after embedding of Hela cells overexpressing PAGFP comprises the following steps:
[0070] (1) Sample fixation
[0071] Hela cells overexpressing PAGFP were fixed by chemical fixation means to obtain fixed PAGFP-labeled biological tissue. Specific steps are as follows:
[0072] At 4 degrees Celsius, Hela cells were soaked in 4% PFA solution for about 12 hours, and then rinsed with PBS solution for 3 times, using 40ml of PBS solution for each cell, and rinsed for 1 hour each time.
[0073] (2) Sample dehydration
[0074] The fixed PAGFP-labeled Hela cells were replaced with ethanol to dehydrate the sample to obtain a dehydrated PAGFP-labeled cell sample. The specific steps are:
[0075] At 4 degrees Celsius, the fixed PAGFP-labeled Hela cells were sequentially passed through 20 ml of gradient ethanol double-distilled water solution, soaked for 2 hours, and dehydrated. The concentration gradient of ethanol double distilled wate...
Embodiment 3
[0084] The method for controlling fluorescence after embedding of Hela cells overexpressing PAGFP comprises the following steps:
[0085] (1) Sample fixation
[0086] Hela cells overexpressing PAGFP were fixed by chemical fixation means to obtain fixed PAGFP-labeled biological tissue. Specific steps are as follows:
[0087] At 4 degrees Celsius, Hela cells were soaked in 4% PFA solution for about 12 hours, and then rinsed with PBS solution for 3 times, using 40ml of PBS solution for each cell, and rinsed for 1 hour each time.
[0088] (2) Sample dehydration
[0089] The fixed PAGFP-labeled Hela cells were replaced with ethanol to dehydrate the sample to obtain a dehydrated PAGFP-labeled cell sample. The specific steps are:
[0090] At 4 degrees Celsius, the fixed PAGFP-labeled Hela cells were sequentially passed through 20 ml of gradient ethanol double-distilled water solution, soaked for 2 hours, and dehydrated. The concentration gradient of ethanol double distilled wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com