1, 5-diaryl-1, 2, 4-triazole compounds and pharmacy use thereof
A technology of triazoles and compounds, applied in drug combinations, organic chemistry, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Embodiment one: the preparation of each compound
Embodiment 1-1
[0122] Example 1-1, 3-(5-(4-methoxyphenyl)-3-methyl-1H-1,2,4-triazol-1-yl)-N-(4-phenylbutyl base) benzamide
[0123] Take compound 4-methoxybenzoic acid (304mg, 2.0mmol) and acetamidine hydrochloride (284mg, 3.0mmol) in N,N-dimethylformamide, under argon atmosphere, add in ice bath HATU (826mg, 2.2mmol) and DIEA (1.39mL, 8.0mmol) were stirred for 5min, then changed to room temperature (25°C) and stirred for 4h. After cooling, the compound 3-hydrazinobenzoic acid methyl ester (1329mg, 8.0mmol) was added Warm up to 80°C with glacial acetic acid (1.14mL, 20mmol), react for 3h, then cool to room temperature, wash the reaction solution twice with saturated aqueous sodium bicarbonate until no bubbles are generated, extract with ethyl acetate, wash the ethyl acetate layer with saturated saline After drying with anhydrous sodium sulfate, the organic phase was evaporated to dryness. After purification by column chromatography, the compound 3-(5-(4-methoxyphenyl)-3-methyl-1H-1,2,4-tri...
Embodiment 2
[0133] Example 2: The compound target verification experiment of the present invention
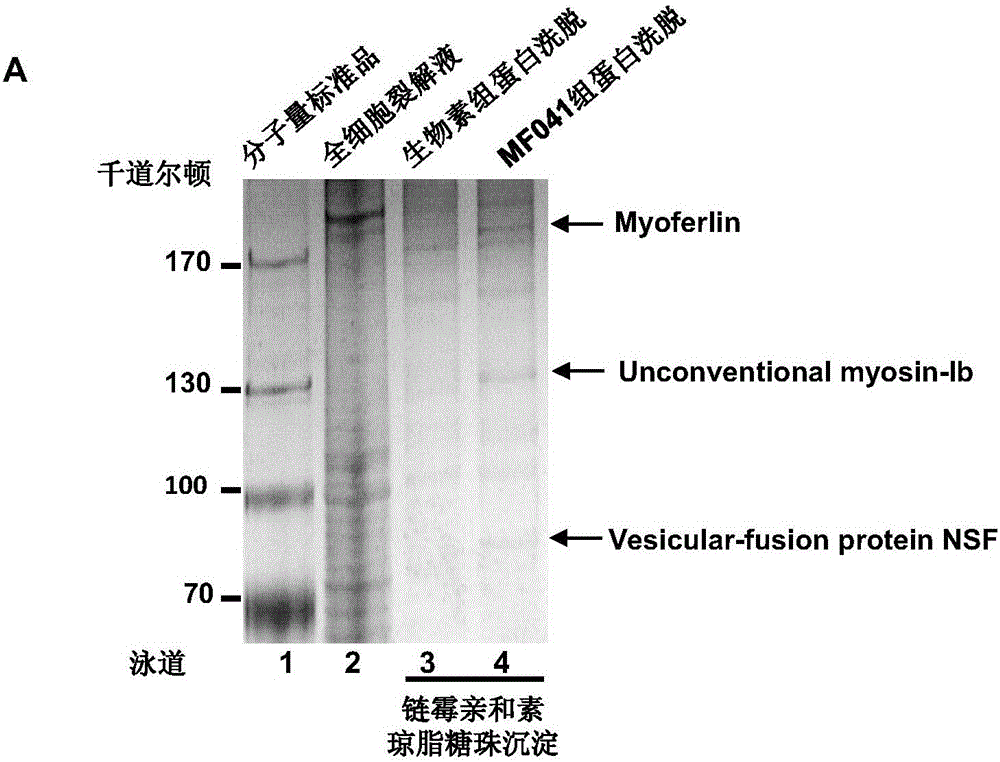
[0134] The cell lysate of breast cancer cell MDA-MB231 was co-incubated with biotin or MF041 coupled with biotin at 4°C for 12h. Then each group was added to the agarose beads of streptavidin and continued to incubate for 2h. The agarose beads were collected by centrifugation, the proteins were eluted with 2× loading buffer, and separated by SDS-polyacrylamide gel electrophoresis. After the polyacrylamide gel was silver-stained, the specific target band was excised and identified by mass spectrometry, and it was found that the compound of the present invention can be effectively combined with myoferlin. Take MF041 as an example: the results of silver staining are as follows: figure 1 As shown, in the target verification binding experiment, it was found that the three proteins only appeared in the biotin-coupled MF041 group, but not in the biotin group, indicating that they are proteins t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com