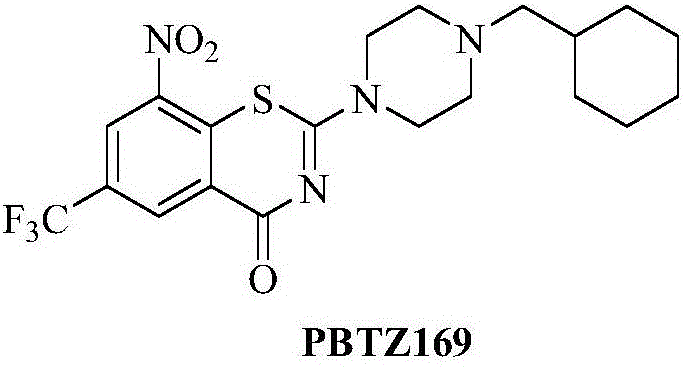
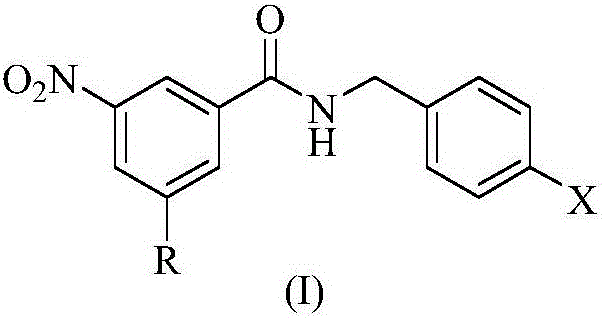
N-benzylbenzamide compounds and preparation method thereof
A technology of benzamide and compound, applied in the field of medicinal chemistry, can solve the problems of weak effect, ineffectiveness and long treatment period of latent MTB, and achieve the effects of low side effects, good curative effect and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 3-nitro-5-trifluoromethyl-N-[4-(4-trifluoromethylpiperidin-1-yl)benzyl]benzamide
[0061] Under nitrogen protection, 3-nitro-5-(trifluoromethyl)benzoic acid (0.23g, 0.1mmol), 4-(4-(trifluoromethyl)piperidin-1-yl)aniline, bis( A mixture of 2-oxo-3-oxazolidinyl)phosphoryl chloride (BOP-Cl, 0.28g, 0.11mmol), triethylamine (0.15g, 0.15mmol) and dichloromethane (25ml) was stirred at room temperature Reaction 2h. After the reaction was completed, it was washed with water (25 ml), and then the organic layer was washed with saturated sodium chloride solution. After concentration, the residue was separated by column chromatography to obtain 0.33 g of off-white solid (70.0% yield), mp: 143-144°C.
[0062] 1 H NMR (500MHz, CDCl 3 )δ9.54(t, J=5.5Hz, 1H), 8.97(s, 1H), 8.66(d, J=9.0Hz, 2H), 7.21(d, J=8.5Hz, 2H), 6.93(d, J=8.5Hz, 2H), 4.44(d, J=5.5Hz, 2H), 3.75(d, J=12.5Hz, 2H), 2.66-2.71(m, 2H), 2.45-2.49(m, 1H), 1.85 (d, J=12.5Hz, 2H), 1.49-1.57 (m, 2H).
[0063] MS...
Embodiment 2
[0065] Example 2 3,5-Dinitro-N-[(4-thiomorpholinyl)benzyl]benzamide
[0066] Same as the preparation method of the compound in Example 1, 3,5-nitrodibenzoic acid reacted with 4-(thiomorpholin-4-yl)aniline to obtain an off-white solid (yield 62.0%), mp: 184-186°C .
[0067] 1 H NMR (500MHz, CDCl 3 )δ9.65(t, J=5.5Hz, 1H), 9.08(d, J=2.0Hz, 2H), 8.95(s, J=2.0Hz, 1H), 7.21(d, J=9.0Hz, 2H) , 6.89 (d, J=9.0Hz, 2H), 4.44 (d, J=5.5Hz, 2H), 3.47-3.50 (m, 4H), 2.63-2.65 (m, 4H).
[0068] MS-ESI(m / z):403.2(M+H) + .
[0069] HRMS-ESI:m / z Calcd.for C 18 h 19 o 5 N 4 S(M+H) + : 403.19172; Found 403.19065.
Embodiment 3
[0070] Example 3 3,5-Dinitro-N-[4-(octahydro-2H-isoindol-2-yl)benzyl]benzamide
[0071] With the preparation method of the compound of Example 1, 3,5-nitrodiylbenzoic acid was reacted with 4-(octahydro-2H-isoindol-2-yl)aniline to obtain an off-white solid (yield 67.1%), mp: 188-189°C.
[0072] 1 H NMR (500MHz, CDCl 3 )δ9.65(t, J=5.5Hz, 1H), 9.08(d, J=2.0Hz, 2H), 8.95(s, J=2.0Hz, 1H), 7.21(d, J=9.0Hz, 2H) ,6.89(d,J=9.0Hz,2H),4.44(d,J=5.5Hz,2H),2.97-2.99(m,4H),1.58-1.53(m,6H),1.38-1.36(m,4H ).
[0073] MS-ESI(m / z):425.4(M+H) + .
[0074] HRMS-ESI:m / z Calcd.for C 22 h 25 o 5 N 4 (M+H) + :424.39263; Found 425.39195.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com