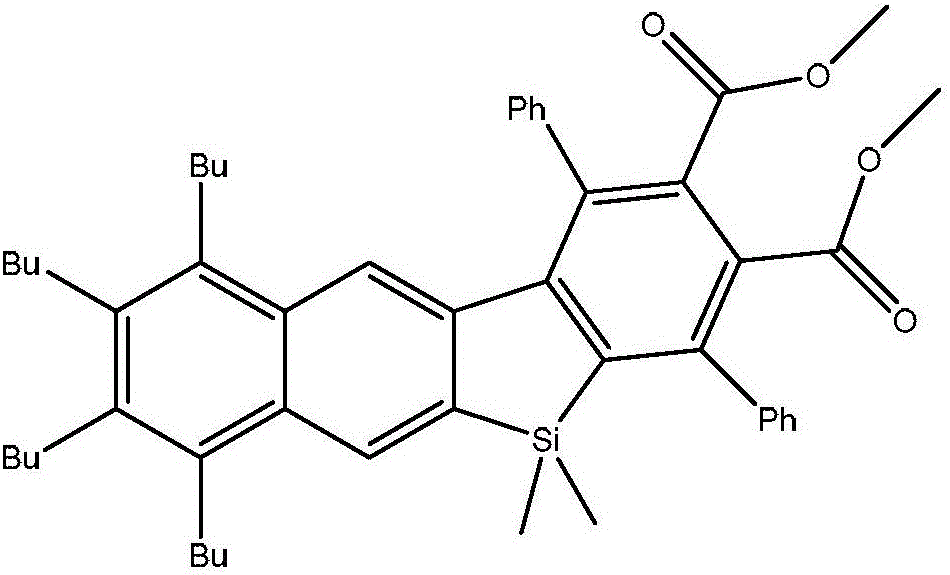
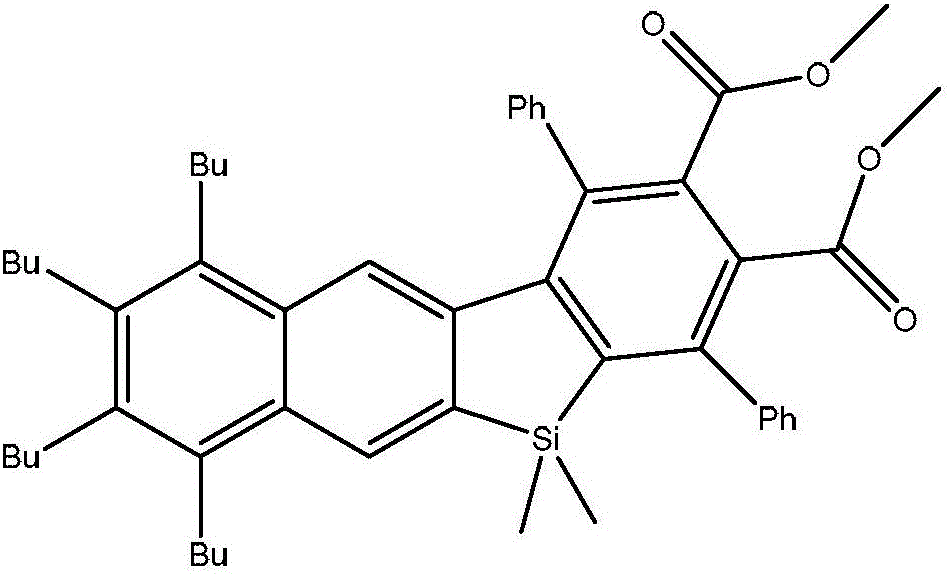
Benzene and naphtho-silole derivative organic photoelectric material and preparation method thereof
A technology of organic photoelectric materials and naphthalene derivatives, which is applied in the chemical field to achieve the effects of mild conditions, low energy consumption and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: prepare a kind of benzene, naphthosilole derivative organic photoelectric material, the steps are as follows:
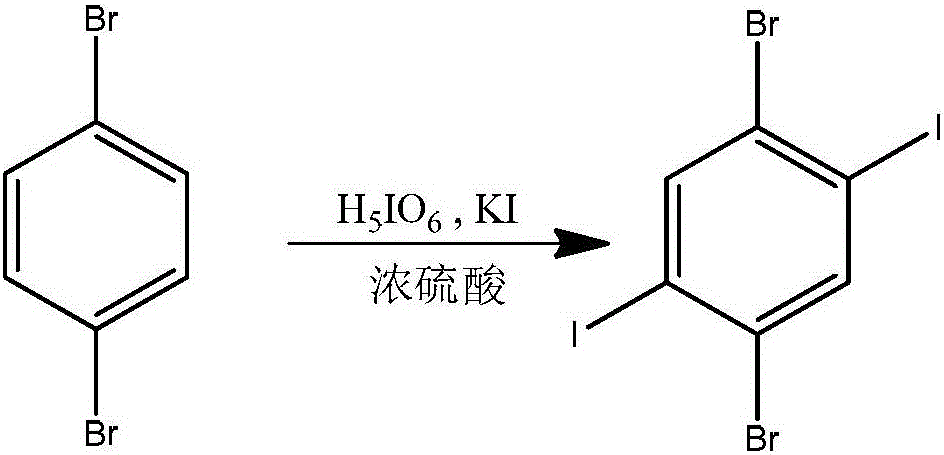
[0023] Step 1. Preparation of 1,4-dibromo-2,5-diiodobenzene: Add 2.66g (11.7mmol) of periodic acid into a 250mL reaction flask, cool down to 0°C, and add 60mL of concentrated sulfuric acid. After stirring, 5.82g (35.1mmol) of potassium iodide was added, and after 15 minutes, 5.52g (23.4mmol) of p-dibromobenzene and 24mL of concentrated sulfuric acid were added successively, and the reaction was carried out at 0°C for 12 hours. Gradually rise to room temperature and react for 10 hours. Pour the reaction solution in the bottle into ice, stir, and filter the obtained filter cake, which is the crude product of 1,4-dibromo-2,5-diiodobenzene, dissolve the filter cake in chloroform, wash with dilute NaOH solution, and extract , the organic phase was dried, concentrated, and recrystallized to obtain 7.87g (yield 69%) of 1,4-dibromo-2,5-diiodobenzene. Th...
Embodiment 2
[0035] Embodiment 2: Prepare a kind of benzene, naphthosilole derivative organic photoelectric material, the steps are as follows:
[0036] Step 1. Preparation of 1,4-dibromo-2,5-diiodobenzene: Add 6.82g (30mmol) of periodic acid into a 1000mL reaction flask, cool down to 0°C, and add 150mL of concentrated sulfuric acid. After stirring, 14.92g (90mmol) of potassium iodide was added, and after 15 minutes, 14.15g (60mmol) of p-dibromobenzene and 60mL of concentrated sulfuric acid were added successively, and the mixture was reacted at 0°C for 10 hours. Gradually rise to room temperature and react for 8 hours. Pour the reaction solution in the bottle into ice, stir, and filter the obtained filter cake, which is the crude product of 1,4-dibromo-2,5-diiodobenzene, dissolve the filter cake in chloroform, wash with dilute NaOH solution, and extract , the organic phase was dried, concentrated, and recrystallized to obtain 16.67g (57% yield) of 1,4-dibromo-2,5-diiodobenzene. The solve...
Embodiment 3
[0042] Embodiment 3: Prepare a kind of benzene, naphthosilole derivative organic photoelectric material, the steps are as follows:
[0043] Step 1. Preparation of 1,4-dibromo-2,5-diiodobenzene: Add 2.66g (11.7mmol) of periodic acid into a 250mL reaction flask, cool down to 0°C, and add 60mL of concentrated sulfuric acid. After stirring, 5.82g (35.1mmol) of potassium iodide was added, and after 15 minutes, 5.52g (23.4mmol) of p-dibromobenzene and 24mL of concentrated sulfuric acid were added successively, and the reaction was carried out at 0°C for 18 hours. Gradually rise to room temperature and react for 15 hours. Pour the reaction solution in the bottle into ice, stir, and filter the obtained filter cake, which is the crude product of 1,4-dibromo-2,5-diiodobenzene, dissolve the filter cake in chloroform, wash with dilute NaOH solution, and extract , the organic phase was dried, concentrated, and recrystallized to obtain 8.326g (yield 73%) of 1,4-dibromo-2,5-diiodobenzene. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com