In-vitro abortive phthisic test method
A technology for active tuberculosis and in vitro detection, which is applied in the field of detection, can solve the problems of poor repeatability of molecular diagnostic methods, false positive test results, and long time consumption, and achieve the effects of wide coverage and practicability, accurate judgment results, and shortened detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Separation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation
[0025] Use fresh heparin lithium or heparin sodium blood collection tubes to aseptically extract 4-5ml of peripheral venous blood from the person to be tested, and invert the blood to mix the anticoagulant and blood. Prepare two 15ml centrifuge tubes, and add physiological saline for injection and lymphocyte separation solution equal to the volume of blood collection respectively. After mixing the fresh heparin anticoagulated blood with normal saline, slowly add it to the separation liquid at a uniform speed. When adding, keep the separation liquid and blood layered, and then centrifuge at 22°C and 1800g for 20 minutes. After centrifugation, PBMCs cells can be seen in the form of clouds layer. Aspirate the PBMCs cell layer into a new 15ml centrifuge tube, make up to 12ml with RPMI-1640 culture medium, and centrifuge at 600g for 10min at 22°C. Pour off the supe...
Embodiment 2
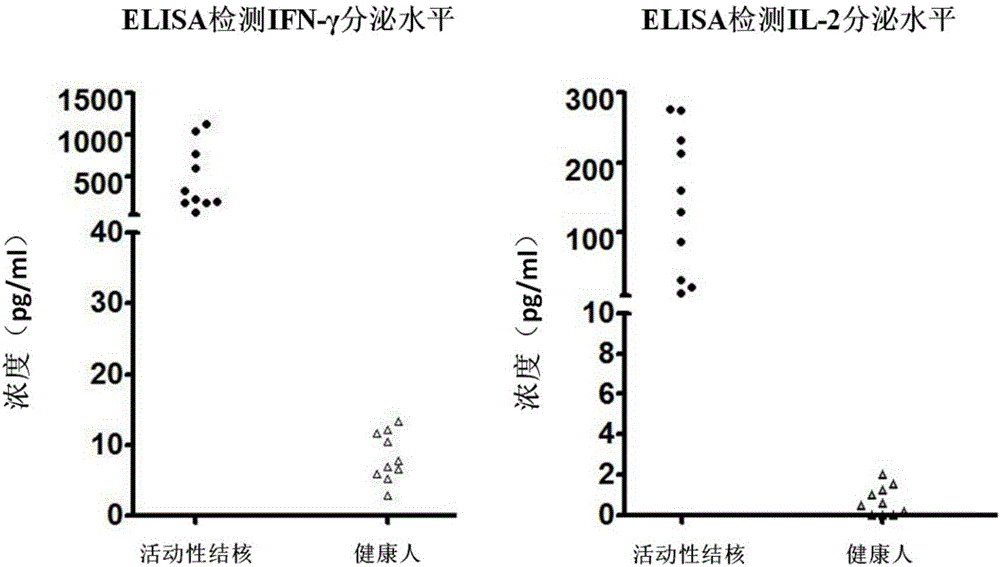
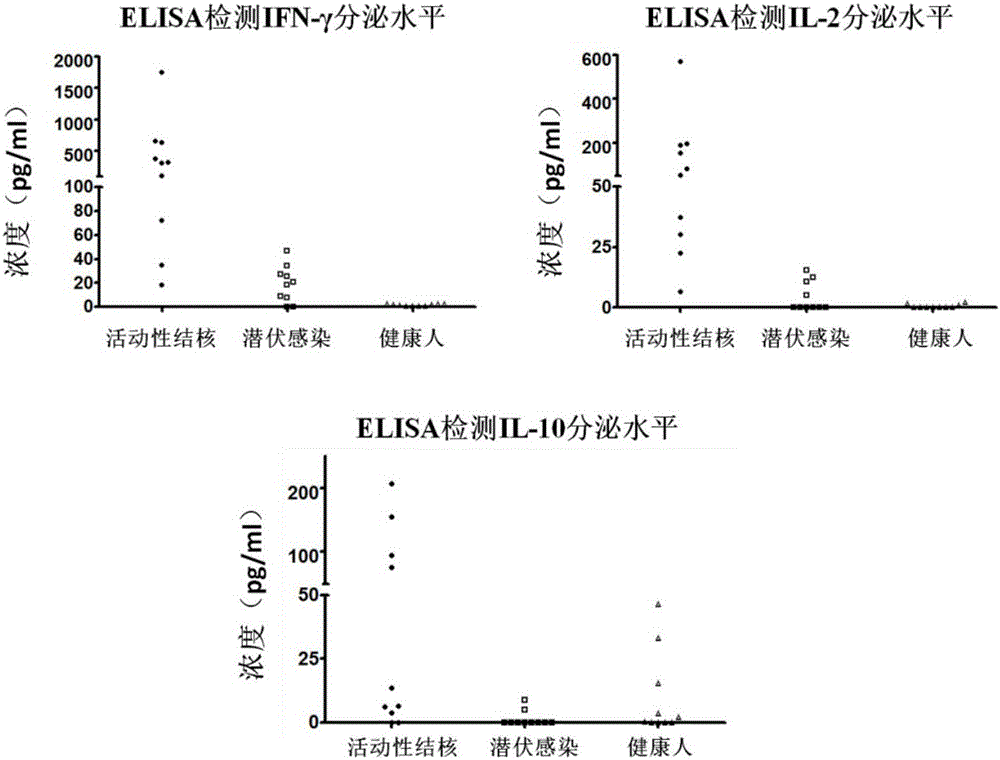
[0026] Example 2 Enzyme-linked immunosorbent assay (ELISA) detects the level of IFN-γ and IL-2 secreted by PBMC after the induction of clinically diagnosed active tuberculosis patients and healthy people
[0027] 1) Select the heparin sodium anticoagulated peripheral venous blood samples of 10 patients clinically diagnosed as active tuberculosis and 10 healthy people, separate PBMCs according to the density stratification method in Example 1, and use serum-free medium after cell counting Dilute PBMCs to 2.5 x 10 6 cells / ml concentration, the cells were seeded into 96-well plates, and 2.5×10 5 Then add specific stimulating antigen (fusion protein CFP-10-ESAT-6-Rv1985c, concentration 2 μg / ml) diluted with serum-free medium, 37°C, 5% CO 2 After incubation for 18 hours under the same conditions, the cell culture fluid was taken to detect IFN-γ and IL-2 cytokines.
[0028] 2) Use the ELISA plate pre-coated with anti-human IFN-γ monoclonal antibody, add the cell culture supernatan...
Embodiment 3
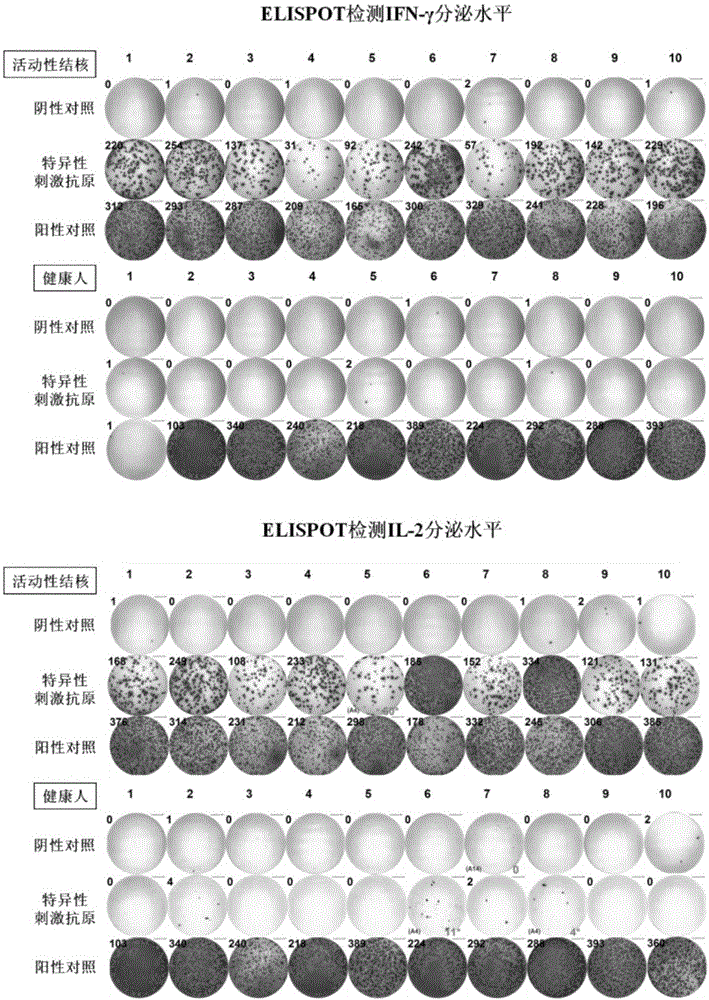
[0031] Example 3 Enzyme-linked immunospot method (ELISPOT) detects the level of IFN-γ and IL-2 secreted by PBMC after the induction of clinically diagnosed active tuberculosis patients and healthy people
[0032]1) Select the heparin sodium anticoagulated peripheral venous blood samples of 10 patients with active tuberculosis and 10 healthy people, separate PBMCs according to the density stratification method in Example 1, and use serum-free medium to dilute it to 2.5×10 6 A / ml concentration for use.
[0033] 2) Select the PVDF membrane detection plate (pre-coated with anti-human IFN-γ monoclonal antibody and anti-human IL-2 monoclonal antibody respectively, and store at 4°C) as the reaction plate, add 2.5×10 5 For each PBMC prepared in the previous step, three detection wells were set for each cytokine in each sample, which were blank control (adding serum-free medium), experimental well (adding specific stimulating antigen for stimulation, the concentration was 2 μg / ml) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com