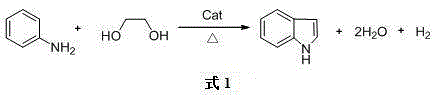
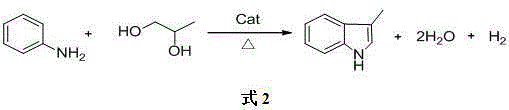
Method for combined production of 3-skatole and indole
A technology of methylindole and indole, which is applied in the field of synthesizing 3-methylindole and indole, can solve the problems of single product structure and low yield of indole, achieve reduced circulation, simple process, and reduced cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Aniline, ethylene glycol, and 1,2-propanediol enter the fixed-bed reactor at a ratio of 5:1:1 (molar ratio), and the catalyst is Ag / SiO 2 , the loading of Ag is 0.5%, the reaction temperature is 250°C, the reaction pressure is normal pressure, the residence time of the gas phase reaction is 0.5 seconds, the volume fraction of hydrogen added in the gas phase feed is 5%, and the volume fraction of water vapor added is 0.5%. After the reaction The gas phase material enters the condenser for condensation, collects the condensate, separates oil from water, and detects the oil phase. The conversion rate of ethylene glycol is 87%, and the yield of indole is 40% (based on hexylene glycol); the conversion rate of 1,2-propanediol is 88%. %, the yield of 3-methylindole is 10% (based on 1,2-propanediol).
Embodiment 2
[0025] Aniline, ethylene glycol, and 1,2-propanediol enter the fixed-bed reactor at a ratio of 7:1:1 (molar ratio), and the catalyst is Ag / SiO 2 , the loading of Ag is 5%, the reaction temperature is 300°C, the reaction pressure is 0.1MPa, the gas phase reaction residence time is 5 seconds, the volume fraction of hydrogen added in the gas phase feed is 5%, the volume fraction of water vapor added is 5%, after the reaction The gas phase material enters the condenser for condensation, collects the condensate, separates oil from water, and detects the oil phase. The conversion rate of ethylene glycol is 95%, and the yield of indole is 72% (based on ethylene glycol); the conversion rate of 1,2-propanediol is 95%. %, the yield of 3-methylindole is 16% (based on 1,2-propanediol).
Embodiment 3
[0027] Aniline, ethylene glycol, and 1,2-propanediol enter the fixed-bed reactor at a ratio of 10:1:1 (molar ratio), and the catalyst is Ag / SiO 2 , the loading of Ag is 8%, the reaction temperature is 350℃, the reaction pressure is 0.5MPa, the residence time of the gas phase reaction is 10 seconds, the volume fraction of hydrogen added in the gas phase feed is 3%, the volume fraction of water vapor added is 15%, after the reaction The gas phase material enters the condenser for condensation, collects the condensate, separates oil from water, and detects the oil phase. The conversion rate of ethylene glycol is 98%, and the yield of indole is 80% (calculated as ethylene glycol); the conversion rate of 1,2-propanediol is 98%. %, the yield of 3-methylindole is 40% (based on 1,2-propanediol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com