In-vivo efficacy evaluation method of EV71 vaccines and antiviral drug screening method
A technology of EV71 and vaccines, applied in biochemical equipment and methods, measurement/inspection of microorganisms, cells modified by introducing foreign genetic material, etc., can solve problems such as limiting research work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0091] The present invention also specifically relates to the following embodiments:
[0092] In one embodiment of the present invention, the present invention relates to the method for constructing R26-hSCARB2 mouse, and it mainly comprises the following steps:
[0093] 1) Using embryonic stem cells to knock in the hSCARB2 gene
[0094]Targeting vectors capable of homologous recombination with gene sites in the genome (mutated target genes, homology arms, resistance genes, etc.) were obtained through molecular carrier construction technology; targeting vectors were introduced into embryonic stem cells by electroporation technology, and challenged The drug-resistant embryonic stem cells were taken, and 4 correctly targeted embryonic stem cell clones were screened out by PCR (see Table 1 for primers) and Southern blotting.
[0095] 2) Microinjection of embryonic stem cells into blastocysts and germline inheritance
[0096] Two of the positive embryonic stem cell clones were s...
Embodiment 1
[0127] Example 1: Construction of hSCARB2 targeting vector
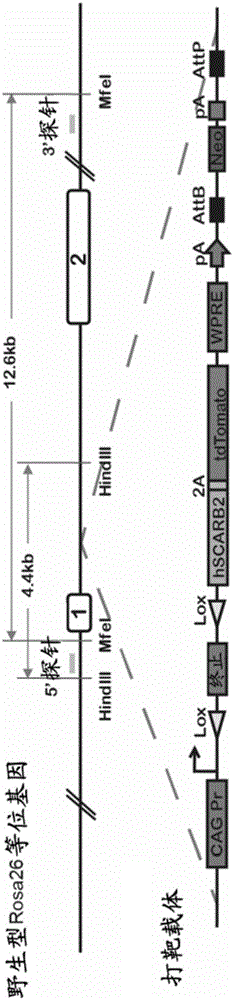
[0128] Rosa26 gene can encode a non-essential nuclear RNA in almost all tissues, and can make the inserted exogenous gene express systemically without affecting the expression of other genes. We inserted the cDNA of hSCARB2 gene (SEQ ID NO:1) into the modified Ai3 targeting vector (Addgene, Madisen L, et al. A robust and high-throughput Crereporting and characterization system for the whole mouse brain. Nat Neurosci13,133-140 , 2010). The Ai3 vector in turn contains a strong CAG promoter, a termination signal flanked by loxP, and a WPRE element that enhances mRNA transcriptional stability. And insert tdTomato reporter gene (SEQ ID NO:11) at the back of hSACARB2, connect with hSCARB2 through 2A short peptide (coded by SEQ ID NO:10) ( figure 1 ). Under the action of Cre recombinase, the transcription termination signal with loxP is cut off, hSCARB2 and tdTomato can be expressed simultaneously, and the 22nd amino aci...
Embodiment 2
[0129] Example 2: Construction of hSCARB2 conditional knock-in mice
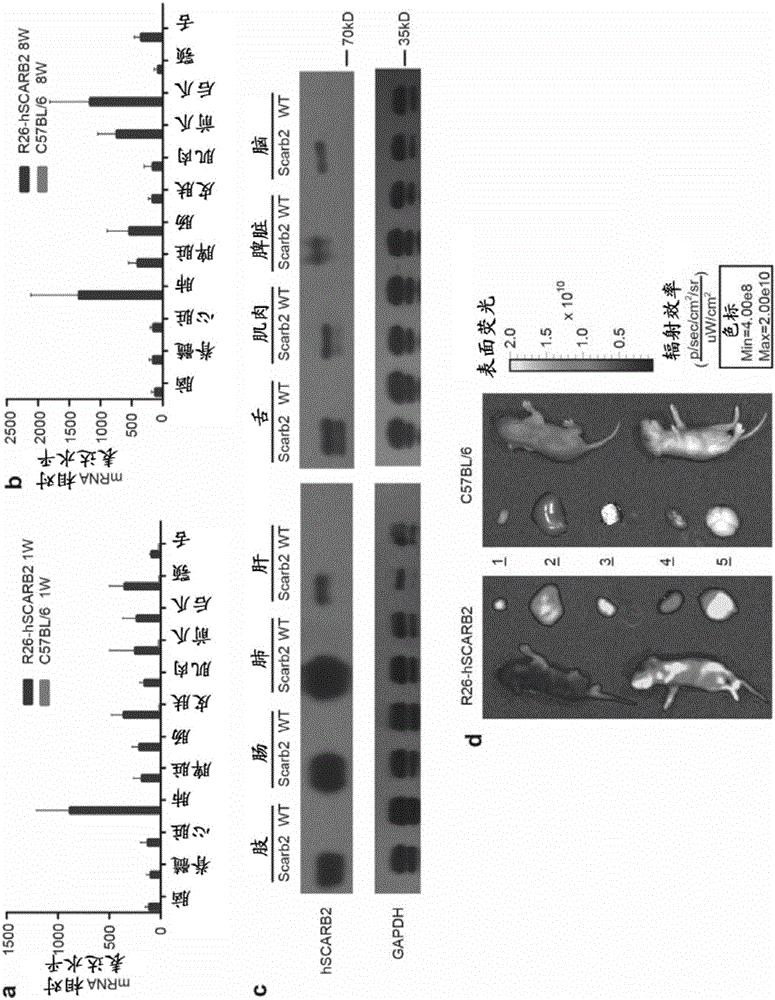
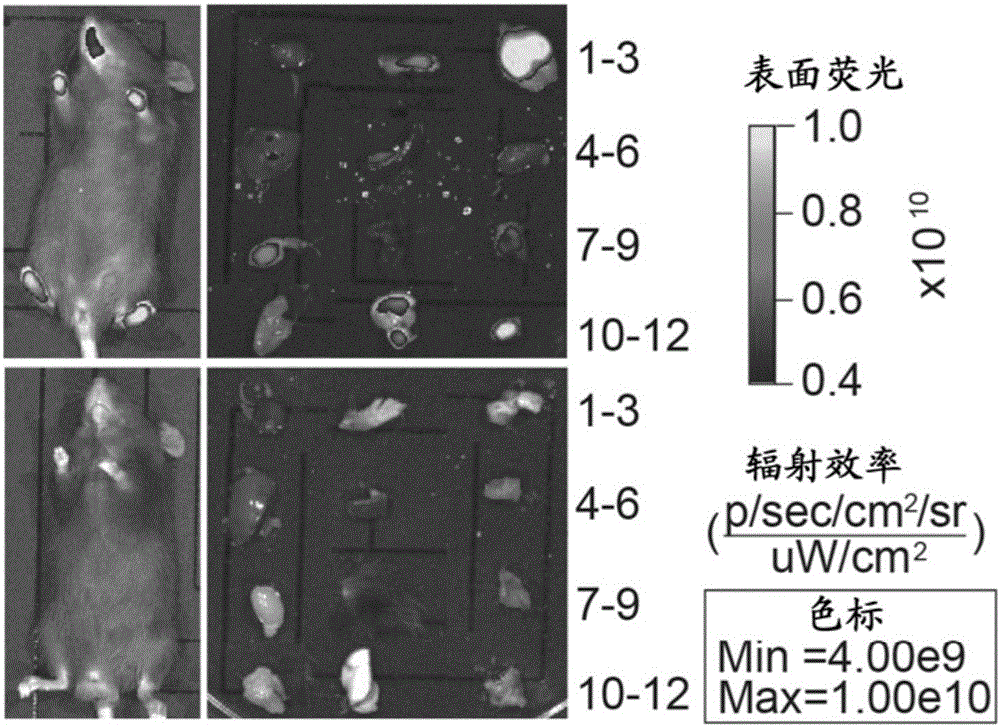
[0130] The targeting vector obtained in Example 1 was transduced into C57BL / 6ES cells by electroporation, and the clones selected by G418 were detected by PCR and southern hybridization to detect 4 correct hSCARB2 gene insertion positive clones. In order to obtain hSCARB2 knock-in mice, we selected one of the positive clones for blastocyst injection. Using BALB / c blastocysts as recipients, 98 blastocysts were injected and transplanted into ICR pseudopregnant mother mice to obtain 8 chimeric mice. Then the chimeric mice were mated with C57BL / 6 mice, and 10 C57BL / 6 background heterozygous gene knock-in mice were obtained completely derived from targeted ES cells through PCR identification. Furthermore, heterozygous mice were mated to obtain homozygous knock-in mice, which were named B6-Gt(Rosa)26Sortm1(CAG-hSCARB2-tdTomato), abbreviated as R26-STOP-hSCARB2 mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com