Enzymatic preparation method for 2, 5-furandicarboxylic acid
A technology for furandicarboxylic acid and enzymatic preparation, which is applied in the field of biological preparation of bulk chemicals, can solve problems such as low FDCA efficiency, and achieve the effects of simple process, high efficiency and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
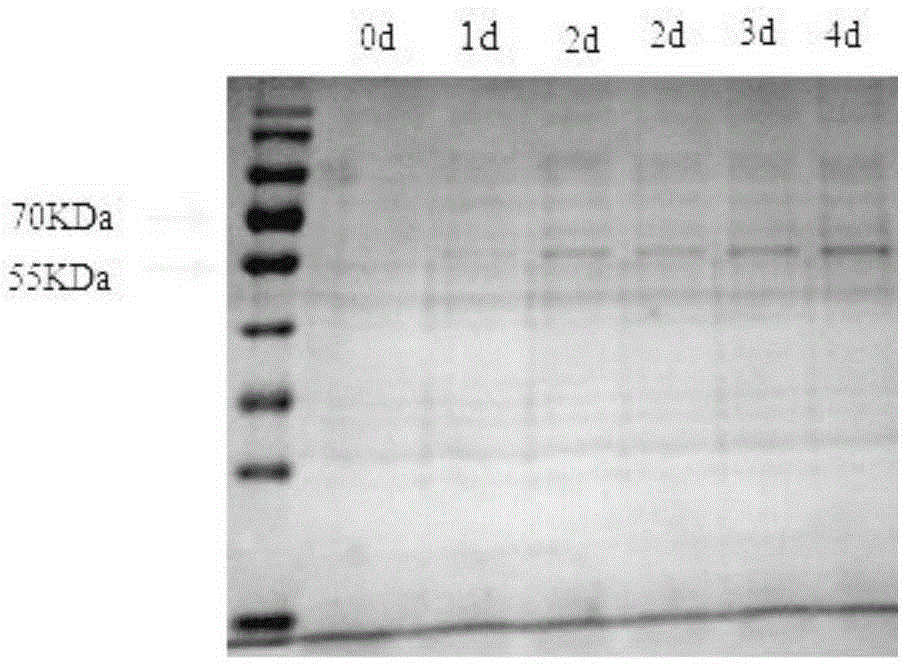
[0029] 1. Synthetic 5-hydroxymethylfurfural oxidase gene (see the HMF oxidase gene sequence for the sequence), after the HMF oxidase gene is cut with restriction endonuclease XhoI and XbaI, it is connected to the same enzyme double-digestion pICZa-A expression vector. Ligate overnight with T4 ligase (the materials in the reaction system consist of 8 μl plasmid, 1 μl reaction buffer, and 1 μl T4 ligase). Take 5 μl of the connection solution to transform into E. coli Top10, and spread it on a bleomycin-resistant plate for overnight culture; take 20 mL of low-salt (0.5% NaCl, V / W) LB culture based on a 50 mL Erlenmeyer flask, add 5 μL of 100 mg / ml bleomycin, insert the transformed strain (E.coli TOP10, Invitrogen Company), shake the strain for 16-18h at 28°C.
[0030] 2. Plasmid extraction: Centrifuge the bacterial liquid obtained in step 1 at 5000 r for 5 min. The supernatant was removed, and the plasmid was extracted with a plasmid kit (Axygen Company, model 05114kai) for pr...
Embodiment 2
[0036] Take 23.8mg of HMF, add 200uL of HMFO prepared in Example 1 to 30mL of 100mM potassium phosphate buffer solution (pH7.16), ventilate with air, 27°C, oil bath, and stir for 30h. HPLC detection shows that the conversion rate of HMF is 99%. Take 16mL of the above reaction solution, and remove water by rotary steaming (40°C) to yellow oily droplets, add 10mL tert-butanol and 10mL ethyl acetate to form a homogeneous mixture, add 200mg lipase Novozym 435, and react 0, 1, 2 , add 165uL H after 3, 4, 5h 2 o 2 (30%), reacted at 40°C for 24h with stirring. Liquid phase detection, FDCA yield was 75%.
Embodiment 3
[0038] Take 23.1mg HMF and dissolve in 30mL 100mM potassium phosphate buffer solution (pH7.16), add 200uL of HMFO prepared in Example 1, ventilate with air, 27°C, oil bath, stir for 30h, and HPLC detection shows that the conversion rate of HMF is 99%. Take 15mL of the above reaction solution, add 6mL of tert-butanol, 1.5mL of ethyl acetate to make a homogeneous mixture, add 225mg of lipase Novozym 435, react for 0, 1, 3, 4, 5h, then add 185uLH 2 o 2 (30%), reacted at 40°C for 24h with stirring. The yield of FDCA detected by liquid phase was 77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com