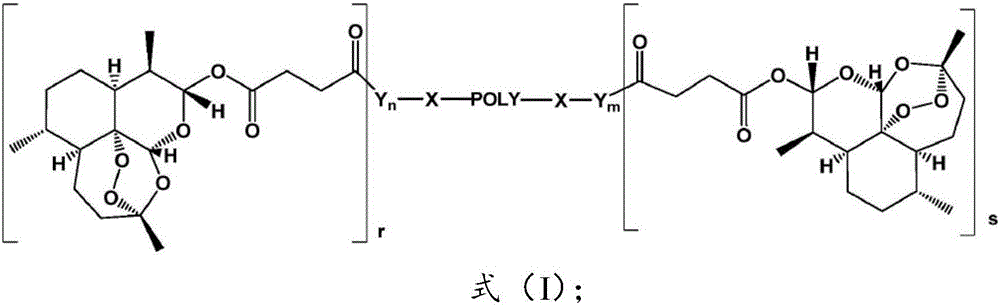
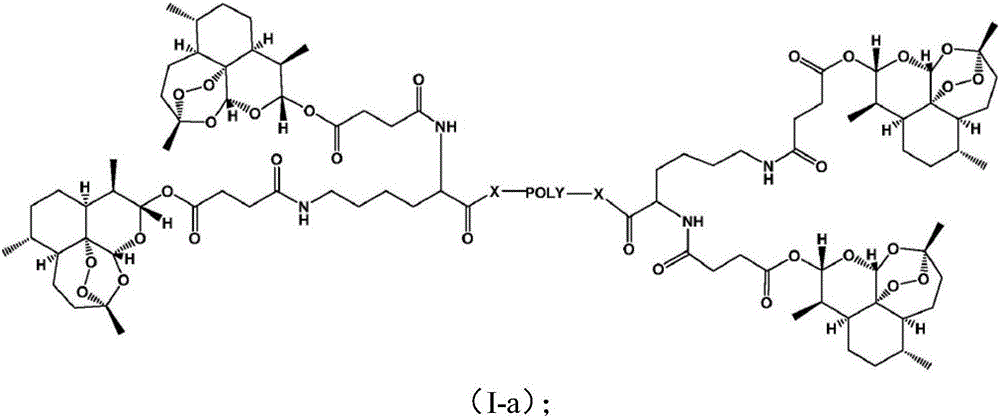
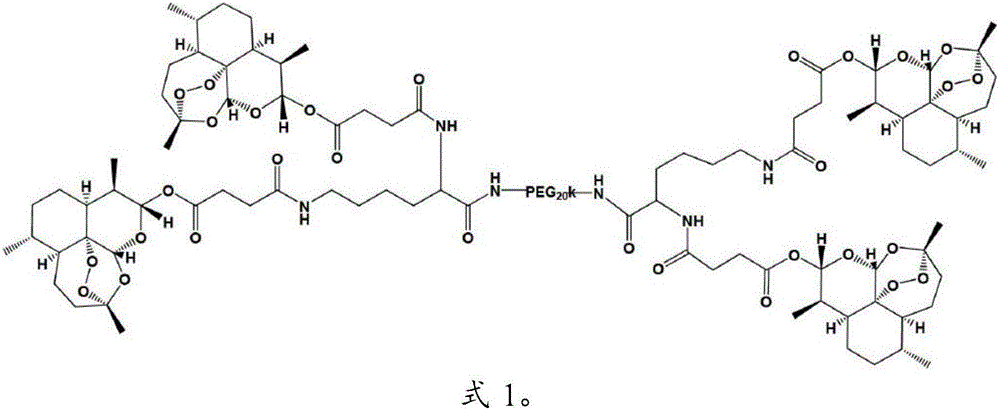
Applications of pegylated artesunate in preparing medicines capable of resisting hepatic pathological changes
A technology of polyethylene glycol artesunate and artesunate, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations, and can solve the problem of anti-hepatic fibrosis of polyethylene glycol artesunate that has not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Study on the Effect of Polyethylene Glycol Artesunate on Rat Hepatic Fibrosis
[0028] 1. Test material
[0029] Polyethylene glycol artesunate, made by Kunming Pharmaceutical Research Institute according to the method disclosed in Example 1 of Chinese patent CN103450468B, has a structure of formula 1; artesunate, Chongqing Huafang Wulingshan Pharmaceutical Co., Ltd.; colchicine tablets , Kunming Pharmaceutical Group Co., Ltd.; hyaluronidase (HA), laminin (LN), type III procollagen (PC-III) kits, Zhongsheng Beikong Biotechnology Co., Ltd.; serum alanine transamination Enzyme (ALT), aspartate aminotransferase (AST), serum albumin (ALB), malondialdehyde (MDA), superoxide dismutase (SOD), hydroxyproline (HyP) kit, HE Dye solution, Nanjing Jiancheng Biological Engineering Co., Ltd.; carbon tetrachloride (AR), Chongqing Chuanjiang Chemical Reagent Factory; precision electronic balance, Beijing Sartorius Balance Co., Ltd.; TDL-5 centrifuge, Shanghai Anting Scientif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com