Hydroxydihydrobovolide in portulaca oleracea, and extraction and separation method of hydroxydihydrobovolide
A technology of hydroxydihydrobeauvolactone and hydroxydihydrobeauvolactone in purslane, applied in food ingredients containing natural extracts, food ingredients, non-central analgesics, etc., can solve the problem of α- Problems such as the separation of β-unsaturated γ-lactone compounds, to achieve the effect of simple and fast operation method, significant anti-tumor activity and anti-HIV activity, and environmentally friendly process methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment provides a kind of hydroxydihydropervolactone, molecular formula is C 11 h 18 o 3 , the chemical structural formula is.
[0029]
[0030] The hydroxydihydropervolactone is named 5-hydroxy-3,4-dimethyl-5-pentanyl-2(5H)-furanone according to the structure, and Table 1 shows the NMR of the unsaturated lactone compound data: 1 H-NMR with 13 C-NMR in CDCl 3 middle.
[0031] Table 1: NMR data of hydroxydihydropervolactone.
[0032]
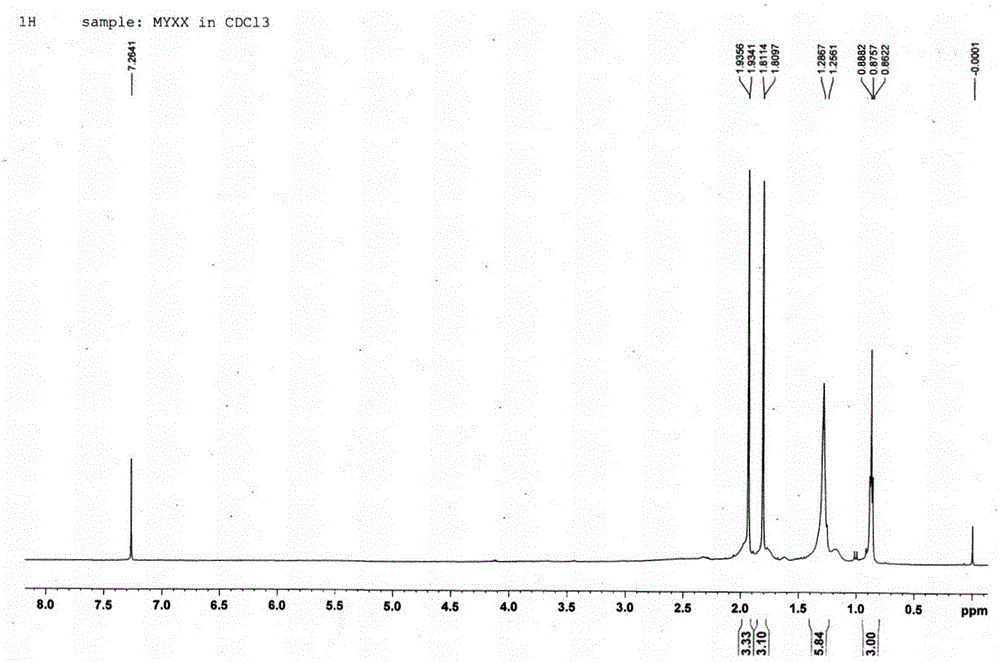
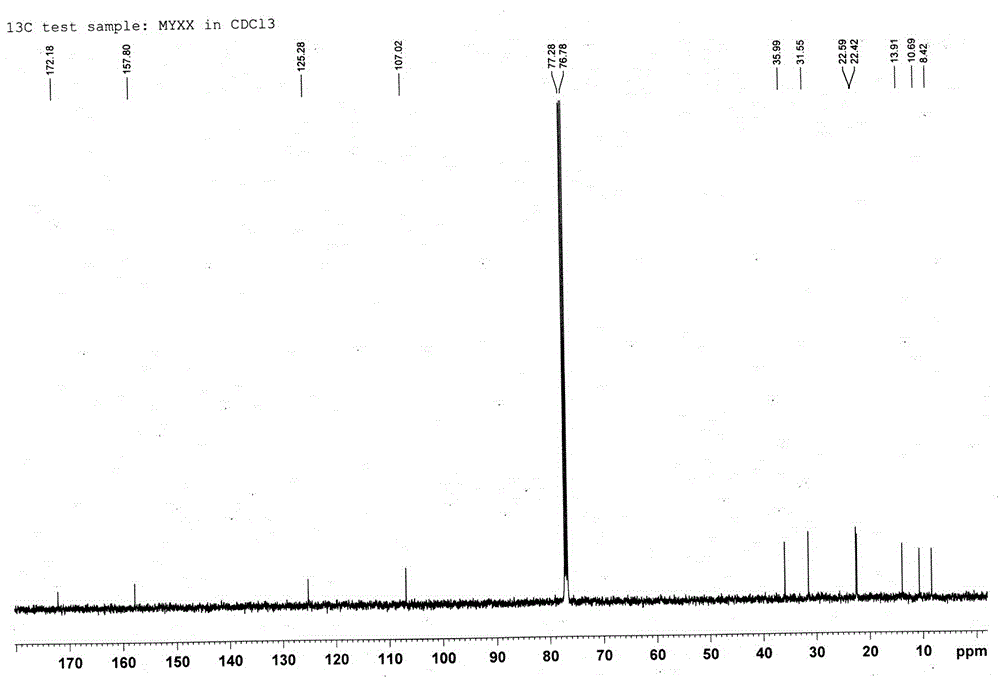
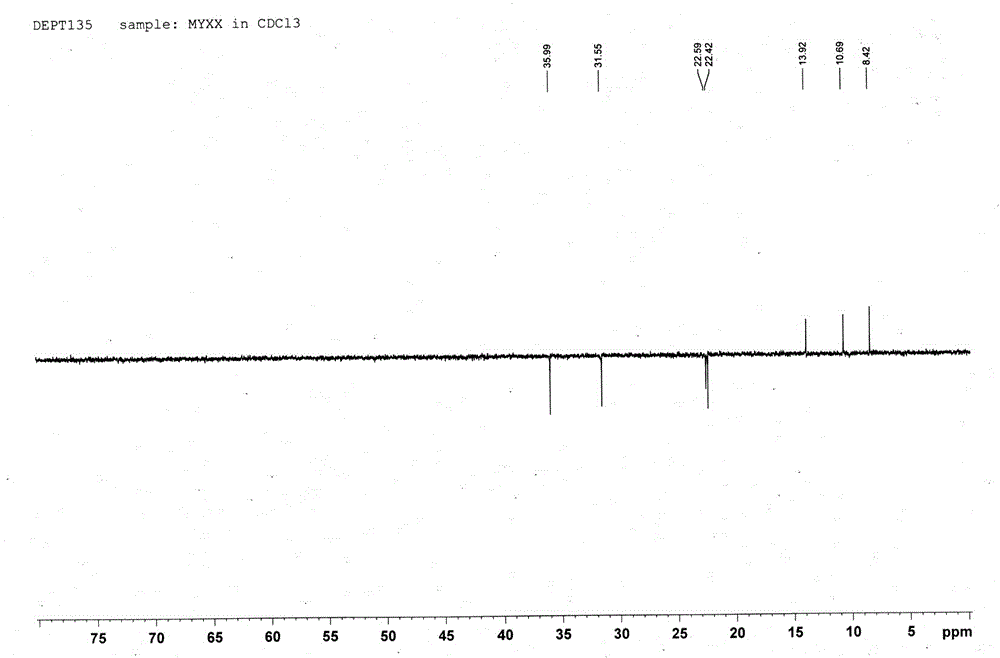
[0033] Compound: Pale yellow oil; HR-ESI-TOF-MS gave m / z: 199.1321 [M+H] + The quasi-molecular ion peak (see figure 1 ), the molecular weight is 198.1404; 1 H-NMR, 13 C-NMR and DEPT data (see Figure 2-4 ), it is speculated that the possible molecular formula of this compound is C 11 h 18 o 3 , the degree of unsaturation is 3; the spectral data are as follows, 1 H-NMR (500MHz, CDCl 3 ) δ: 1.96 (1H, br, H-6a), 1.93 (3H, d, J=0.75Hz, H-11), 1.81 (3H, d, J=0.85 Hz, H-12), 1.77 (1H, br, H-6b), 1.29 (2H, m, H-8,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com