Method of extracorporeal induction, proliferation and cryopreservation of immune cells
An immune cell and cryopreservation technology, applied in the biological field, can solve the problems of culture system safety risks, natural killer cell culture and cryopreservation methods need to be improved, expansion multiples and poor tumor killing effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
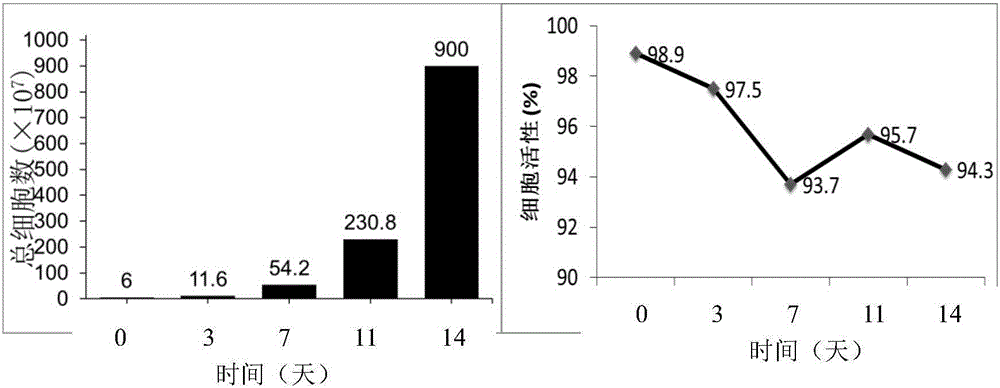
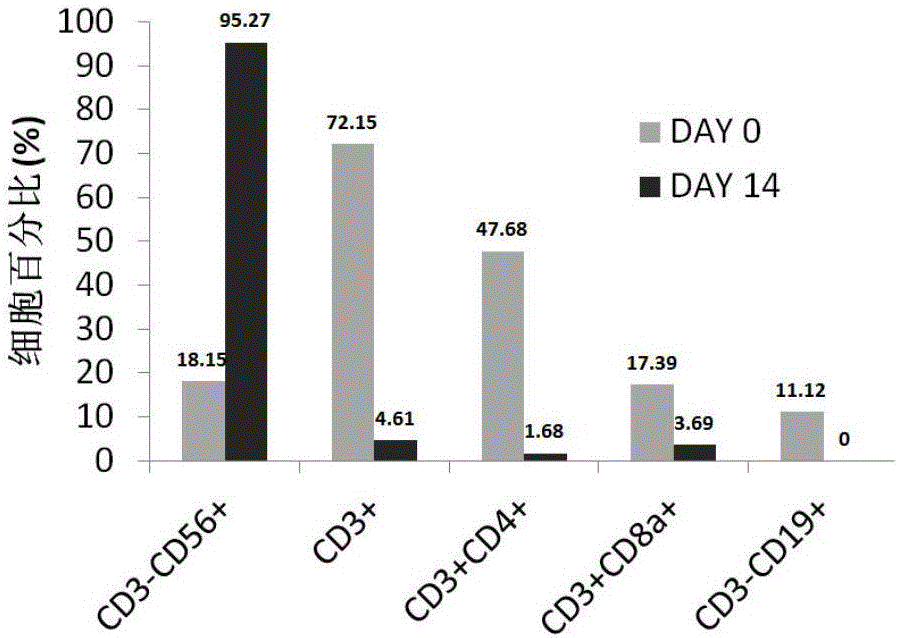
[0077] Using the method for inducing expansion of immune cells in the embodiment of the present invention, the isolated mononuclear cells are induced and expanded into immune cells, and the activity of the immune cells is detected.
[0078] 1. Experimental method
[0079] 1. Prepare anti-human CD16 coated T75 vials
[0080] 1.1 Add 5 mL of 2.5 μg / mL anti-human CD16 monoclonal antibody dissolved in medical normal saline into a sterile culture bottle, shake the culture bottle gently to make the antibody cover the culture surface, and overnight at 4°C in the dark.
[0081] 1.2 Recover the antibody coating solution before use, wash the culture bottle once with 5 mL of normal saline, and then use 5 mL of T cell expansion medium (OpTmizer TM CTS TM T-cell expansion SFM) and washed once.
[0082] 2. Collect peripheral blood, separate peripheral blood plasma and mononuclear cells
[0083] 2.1 Collect about 100ml of human peripheral blood with a sterile blood collection bag added w...
Embodiment 2
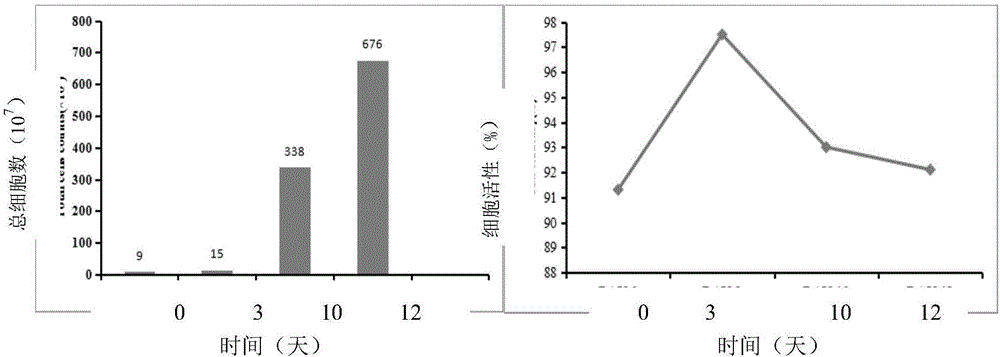
[0126] In this example, 6 kinds of cryopreservation solutions without plasma and 6 kinds of cryopreservation solutions containing plasma were used respectively to freeze the NK cells induced and expanded in vitro in Example 1, and compare the cryopreservation effects so as to observe the effect of plasma on Effects of cryopreservation of NK cells, and the possibility of reducing DMSO concentrations to reduce cytotoxicity. details as follows:
[0127] 1. Use plasma-free cryopreservation medium to freeze NK cells
[0128] 1.1. NK cell cryopreservation and recovery methods:
[0129] The NK cells obtained on the 10th day of in vitro induction and expansion in Example 1 were cryopreserved in six cryopreservation solutions containing HSA, and the components of the six cryopreservation solutions were as follows:
[0130] Freezing solution 1: 5 vol% DMSO+10 vol% HSA;
[0131] Freezing solution 2: 5 vol% DMSO+15 vol% HSA;
[0132] Freezing solution 3: 10% by volume DMSO + 10% by vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com