Method for preparing lactose-N-disaccharide
A technology of lactose and galactose phosphate, applied in the field of preparation of lactose-N-disaccharide, can solve the problems of no benefit to human health, the activity and effect cannot be compared with that of breast milk oligosaccharide, and the function of breast milk oligosaccharide cannot be simulated, and the cost is achieved. Low, high utility value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] A kind of preparation method of lactose-N-disaccharide
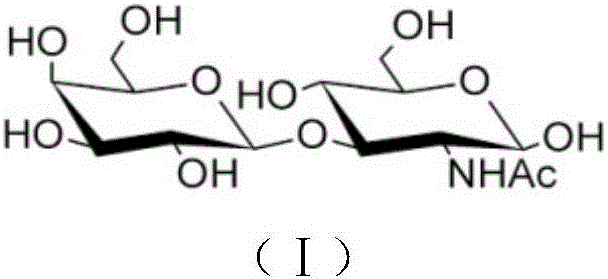
[0024] 1 μM-2M 1-galactose phosphate, 1 μM-2M N-acetylglucosamine, 1 μM-2M phosphate buffer (pH 3-9), 0.01-10000U lactose-N-disaccharide phosphorylase in 5- After reacting at 50°C for 2 minutes to 1 month, lactose-N-disaccharide (Gal-β1,3-GlcNAc) can be produced, and the structural formula is (I). The pure lactose-N-disaccharide product can be obtained after the reaction solution is concentrated, refined and crystallized. The molecular weight of the product is 383 as detected by mass spectrometry, and its structure is confirmed as lactose-N-disaccharide by nuclear magnetic resonance detection. The yield of lactose-N-disaccharide can reach 87%.
[0025]
Embodiment 1
[0026] Embodiment 1 A kind of preparation method of lactose-N-disaccharide
[0027] Phosphorylate 10mM sucrose, 10mM uridine diphosphate glucose, 50U sucrose phosphorylase, UDP-glucose hexose phosphate uridyltransferase, UDP-glucose epimerase, 100U lactose-N-disaccharide Enzyme, 10mM N-acetylglucosamine and 100mM phosphate buffer (pH 6-7) are placed in the same reaction system, and after the reaction system is reacted at 30°C for 10 days, lactose-N-disaccharide can be generated. The pure lactose-N-disaccharide product can be obtained after the reaction solution is concentrated, refined and crystallized. The molecular weight of the product is 383 as detected by mass spectrometry, and its structure is confirmed as lactose-N-disaccharide by nuclear magnetic resonance detection. The yield of lactose-N-disaccharide reached 80%.
Embodiment 2
[0028] Embodiment 2 A kind of preparation method of lactose-N-disaccharide
[0029] 1M sucrose, 1M uridine diphosphate galactose, 10000U immobilized sucrose phosphorylase, UDP-glucose epimerase, UDP-glucose hexose uridine phosphate immobilized on the sodium alginate carrier Acyltransferase, 10000U lactose-N-disaccharide phosphorylase, 2M N-acetylglucosamine and 1M phosphate buffer (pH 8-9) were placed in the same reaction system, and the reaction system was reacted at 50°C After 20 minutes, lactose-N-disaccharide can be produced. The pure lactose-N-disaccharide product can be obtained after the reaction solution is concentrated, refined and crystallized. The molecular weight of the product is 383 as detected by mass spectrometry, and its structure is confirmed as lactose-N-disaccharide by nuclear magnetic resonance detection. The yield of lactose-N-disaccharide was about 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com