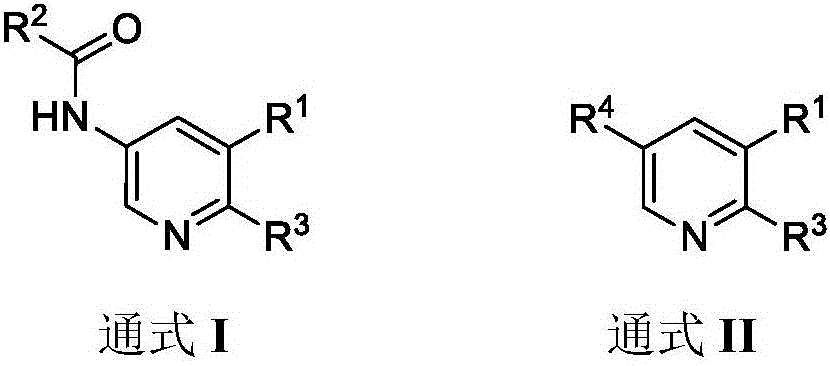
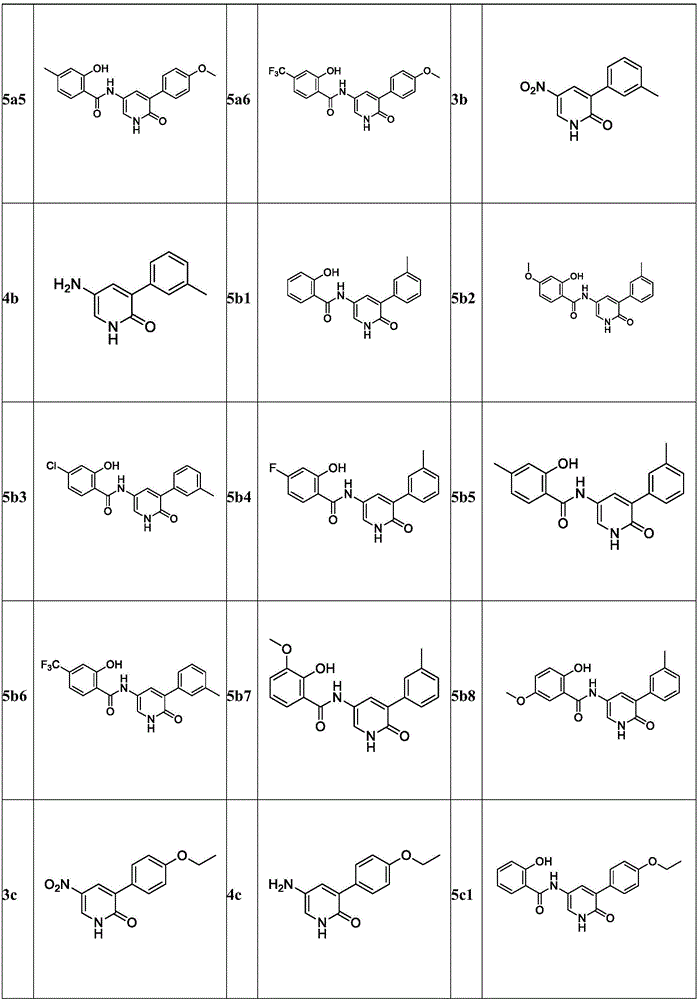
Pyridone derivative, and preparation method and application thereof
A kind of technology of pyridone and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1. Preparation of compound 2
[0056] Take a 1L single-necked bottle, dissolve 5-nitropyridin-2-ol (5g, 35.7mmol) in 500mL of water, slowly add N-bromosuccinimide (3g, 35.7mmol), and stir at 25°C for 3 hours Thin-layer chromatography detected that the reaction was complete, suction filtered, washed with water (100 mL), washed with petroleum ether (50 mL), dried, and weighed to obtain 7.09 g of a light yellow solid with a yield of 90%.
[0057]
[0058] Compound 2 spectral data: 1 H-NMR (400MHz, CD 3 OD) δppm: 8.68(d, 2H, J=2.8Hz), 8.66(d, 2H, J=2.8Hz).
Embodiment 2
[0059] Embodiment 2. Preparation of compound 3a
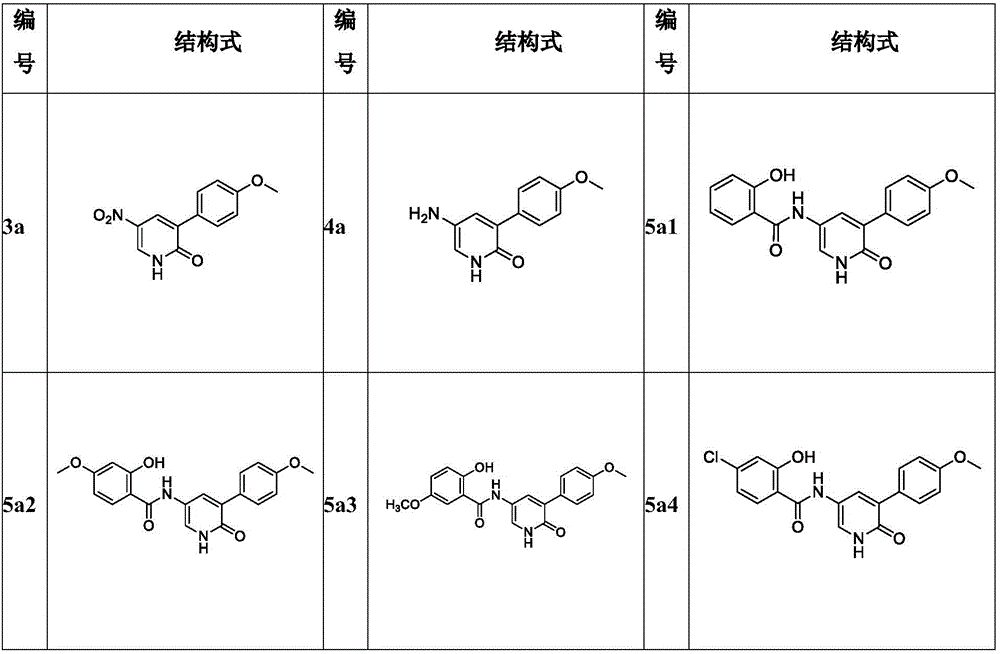
[0060] Take a 100mL anaerobic reaction flask, dissolve compound 2 (2g, 9.13mmol) and p-methoxyphenylboronic acid (1.53g, 10.05mmol) in water (15mL) and a mixed solution of 1,4 dioxane (45mL) In , add potassium carbonate (3.79g, 27.4mmol), replace the nitrogen, quickly add tetrakis triphenylphosphine palladium (0.63g, 0.55mmol); replace the nitrogen again, reflux reaction at 100°C. After the reaction, part of 1,4-dioxane was rotary evaporated, water (60mL) was added, extracted with ethyl acetate (25mL x 3), the organic phases were combined, washed with saturated brine (50mL x 3), dried over anhydrous sodium sulfate, Concentrate and wash with methanol to obtain 1.2 g of yellow solid 3a with a yield of 53% and a melting point of 212-215°C.
[0061]
[0062] Spectral data of compound 1: 1 H-NMR (400MHz, DMSO) δppm: 12.80(s, 1H), 8.62(d, J=3.1Hz, 1H), 8.15(d, J=3.0Hz, 1H), 7.72(d, J=8.8Hz, 2H), 6.99(d, J=8.8Hz, 2H), 3.80(s, 3H...
Embodiment 3
[0063] Embodiment 3. Preparation of compound 4a
[0064] Compound 3a (300 mg, 1.22 mmol) was dissolved in 12 mL of methanol, 10% palladium on carbon (65 mg, 10%) was added to replace the hydrogen, and stirred overnight at 25°C. After the reaction was completed, methanol (20 mL) was added, filtered with diatomaceous earth, washed with methanol (15 mL x 2), and the filtrate was collected and concentrated to obtain 220 mg of tan solid compound 4a with a yield of 83%.
[0065]
[0066] Spectral analysis data of compound 4a: ESI-MS: 217.5[M+H] + ,433.6[2M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com