Purified renaturation method of L-aspartic acid oxidase inclusion bodies
A technology for purification of aspartate oxidase and inclusion bodies, which is applied in the field of bioengineering, can solve problems such as restricted application, low yield, and inactivity, and achieve the effects of saving the use of FAD, reducing economic costs, and avoiding the loss of FAD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
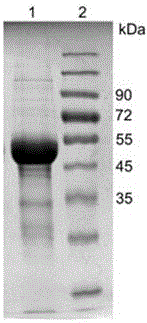
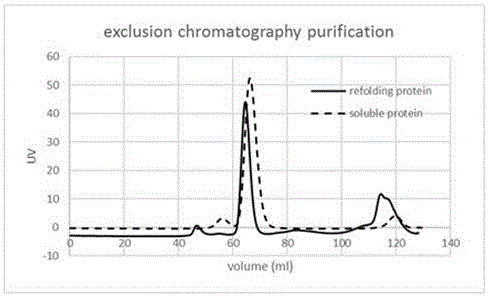
[0047] After the codon optimization of the L-aspartate oxidase gene derived from the thermophilic archaea Sulfolobustokodaii, the target gene was synthesized through the whole gene, connected to the pET-28a vector through molecular cloning, and then transformed into the E. coli host for expression. Most of the products are in the form of inclusion bodies. Therefore, the method of this experiment is used to purify and refold inclusion bodies to obtain active and high-purity L-aspartate oxidase. Specific steps are as follows:
[0048] Step 1. Centrifuge the induced expression strain at 6000 rpm for 15 minutes, remove the supernatant and collect the bacteria. Wash the cells twice with buffer A (50mMTris-HCl, 100mMNaCl, pH8.0), then continue to use buffer A to suspend the cells, use high-pressure crushing, and cycle crushing at 4°C for three times, centrifuge at 15000rpm for 50 minutes, and collect Inclusion body precipitation.
[0049] Step 2: Add 50ml of buffer B (50mM Tris-HC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com