Non-integrative reprogramming method of peripheral blood mononuclear cell for MERRF patient
A technology of peripheral blood and nuclear cells, applied in the field of cell biology and biomedicine, can solve the problems that pluripotent stem cells cannot be fully studied with MERRF, cannot fully simulate patients, and genome changes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
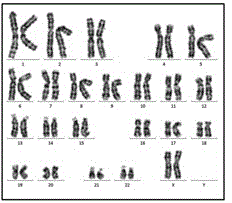
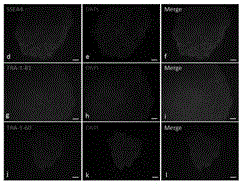
[0020] On the premise of obtaining informed consent, 5 mL of fresh peripheral blood was collected from an 11-year-old MERRF patient in Nanjing Maternal and Child Health Hospital. The patient had been diagnosed with mtDNA 8344A→G mutation by next-generation sequencing. PBMCs were separated with Ficoll lymphocyte separation medium, resuspended in cell freezing medium containing 10% dimethyl sulfoxide (DMSO), and stored in liquid nitrogen. The resulting PBMCs were subjected to non-integrating reprogramming by the Sendai virus method.
[0021] The specific steps are: resuscitate the frozen PBMC, use PBMC complete culture medium (containing SCF (100 ng / μL), Flt-3 (100 ng / μL), IL-3 (20 ng / μL), IL-6 ( 20 ng / μL) StemPro-34 medium) to adjust the cell density to 1×10 6 / mL, seeded into 24-well cell culture plate, 37°C, 5% CO 2 , cultured continuously for 4 days under saturated humidity conditions, and the medium was changed every day. Live cell staining counts were performed with try...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com