Novel exenatide analogue and use thereof
A technology of exenatide and analogues, applied in the field of new exenatide analogues, can solve the problems of excessive reagents and materials, short half-life, many reaction steps, etc., and achieve excellent hypoglycemia, continuous hypoglycemia, prevention or the effect of treating diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Embodiment 1: the preparation method of Ex4(1-32) K-Cap
[0130] The Ex4(1-32) K-Cap of the present invention comprises a lysine-fatty acid (Lysine-fatty acid) added to the sequence of serine at the 32nd position from the N-terminal of exenatide of the following chemical formula I A peptide of 33 amino acids.
[0131] Chemical formula Ⅰ:
[0132] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-trp- Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser
[0133] In order to prepare Ex4 (1-32) K-Cap of the present invention, Fmoc-Lys (dde) and dimethylformamide (DMF, Dimethylformamide) are accumulated in trityl resin (trityl resin) to prepare Fmoc-Ser (tBu )-trityl resin. Added N,N-dimethylformamide (N,N-Dimethylformamide; DMF) containing 20% piperidine to the above Fmoc-Ser(tBu)-trityl resin and containing Fmoc-Pro-OH and hydroxybenzene N, N-dimethylformamide of triazole (HOBt, hydroxy-benzotriazole) to prepare Fmoc...
Embodiment 2
[0148] Example 2: Preparation and Effect Analysis of Short Exenatide-4 Analogs
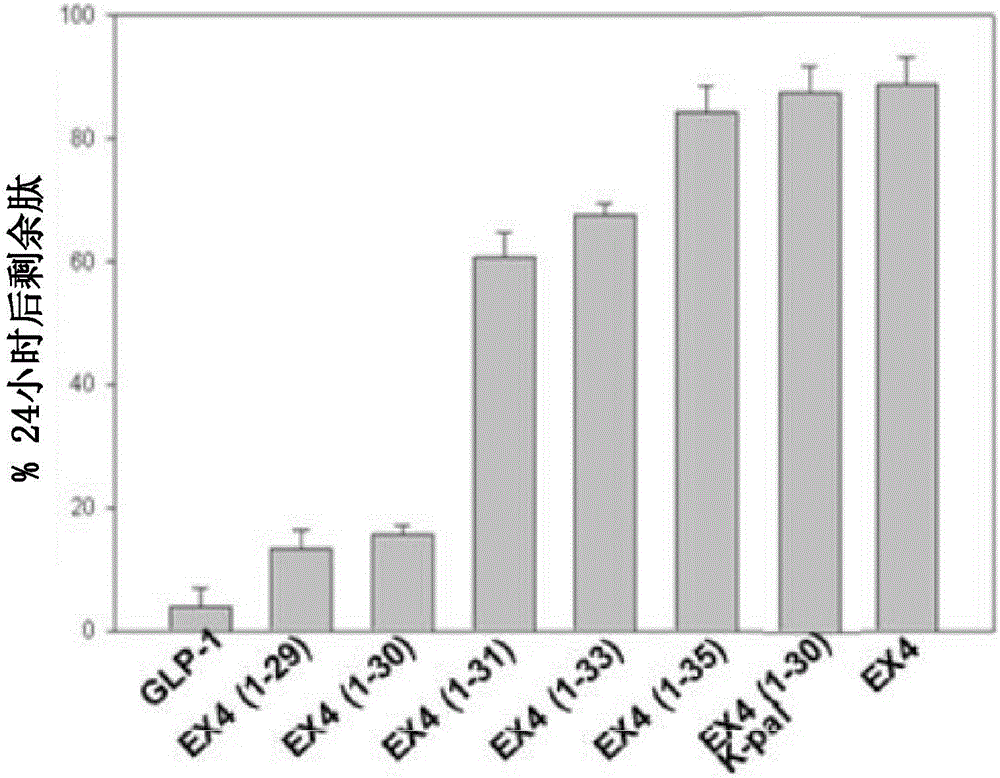
[0149] Preparation of short exenatide-4 analogues and their stability tests against NEP24.11
[0150] The C-terminus of Exenatide-4 has a 9-AA C-terminal sequence (nine-AAC-terminal sequence) that Glucagon-like Peptide-1 does not have, so it is not easy to decompose into neutral peptides such as NEP24.11 Neutral endopeptidase (reference: Doyle ME et al., Regulatory Peptides Volume 114, Issues 2-3, 15 July 2003, Pages 153-158).
[0151] In order to test the stability of NEP24.11, which is a peptidase, the sequence of the C-terminus of the conventional exendin-4 was changed, and various short exendin-4 (short -exenatide-4) analogs.
[0152] (a) Preparation method of Ex4(1-29)
[0153] To prepare Ex4(1-29), accumulate Fmoc-Gly-OH, N,N-diisopropylethylamine (DIEA, N,N-Diisopropylethylamine) and dimethylformamide in trityl resin to prepare Fmoc -Gly-trityl resin. Add N,N-dimethylformamide containi...
Embodiment 3
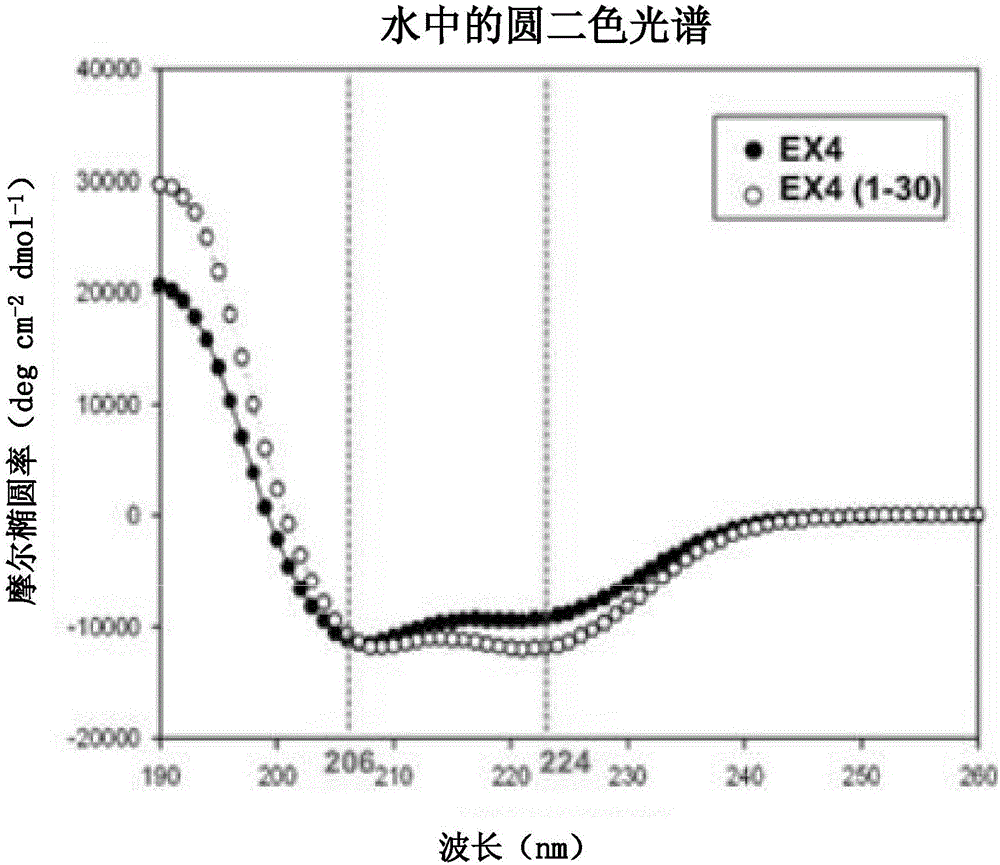
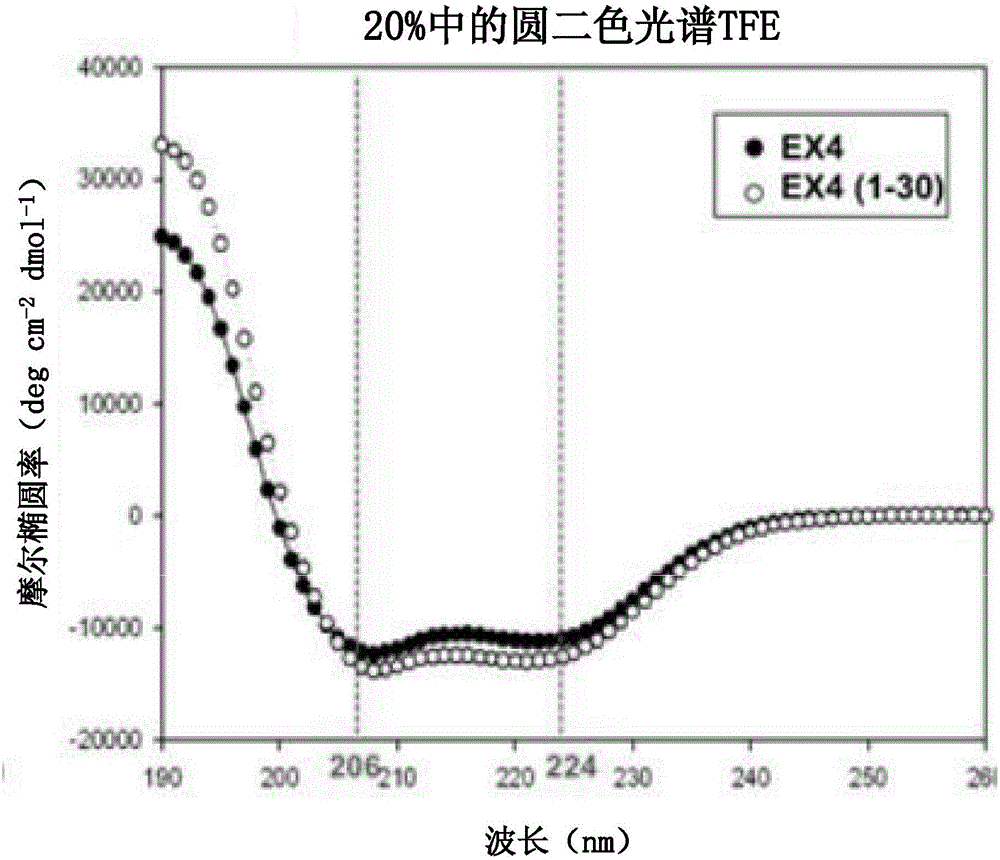
[0197] Embodiment 3: the characteristic analysis of Ex4 (1-32) K (Cap)
[0198] Ex4(1-32)K(Cap)(subcutaneous) glucose tolerance test using diabetic model mice
[0199] After making male db / db mice (6-week-old to 12-week-old) fast for 18 hours, feed them to fasting mice at concentrations of 0.005 nmol / kg, 0.01 nmol / kg, 0.1 nmol / kg, 1 nmol / kg and 5 nmol / kg, respectively. The diabetic model mice were administered (subcutaneously) short exenatide-fatty acid conjugated Ex4(1-32)K(Cap), and 30 minutes later, glucose (1.5 g / kg) was intraperitoneally administered. After 0 minutes, 20 minutes, 40 minutes, 60 minutes, 90 minutes, 120 minutes, and 180 minutes, blood was collected from the tail end of the mice, and blood glucose was measured with a blood glucose meter. As a result, the concentration-dependent hypoglycemic effect of Ex4(1-32)K(Cap) was confirmed ( Figure 13a and Figure 13b ).
[0200] For comparison with Exenatide as a positive control group, Ex4(1-32)K(Cap) and Exen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com