A photosensitive drug sensitive to weak light and preparation method thereof
A photosensitive drug and weak light technology, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of insufficient excitation ability of photosensitizers, limited application range of photodynamic therapy, low light intensity, etc., to achieve Fast speed, high output, and improved effect in hypoxic environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
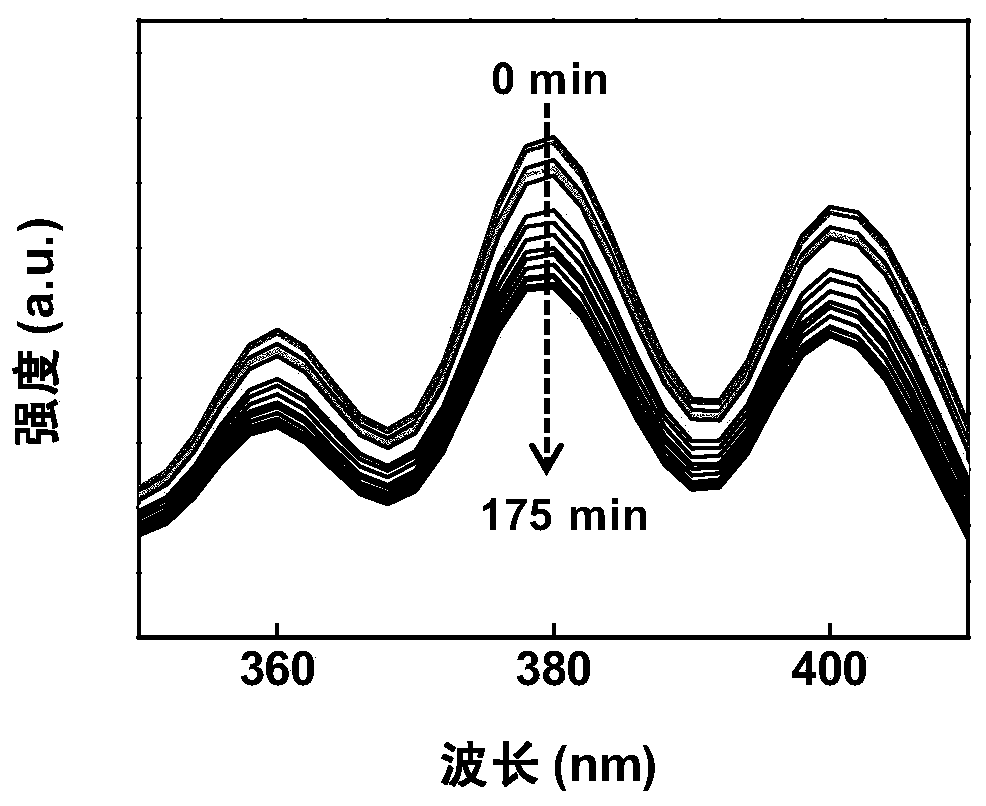
[0043] The concentration of RB contained in the configuration is 0.01mg·mL under the condition of avoiding light -1 3.5 mL of Hb-RB solution, with 70 μL of 1 mg·mL -1 After the DMSO solution of 9,10-Diphenylanthracene (DPA) was mixed evenly, it was placed in a closed quartz cuvette. Place the cuvette at an intensity of about 50μW / cm 2 Under the weak light irradiation, the absorbance of the system in the range of 350-410nm was measured by a UV-visible spectrophotometer, measured every 5 minutes of irradiation, and the change of the absorbance at 380nm was compared.
[0044] DPA is a commonly used chemical trapping agent, which can quickly react with singlet oxygen to form a stable inner oxide, and the content of singlet oxygen can be measured by measuring the decrease of its absorbance at 380nm. Such as figure 1 As shown, it can be seen that with the prolongation of the irradiation time, the absorbance of the system at 380nm gradually weakens, indicating that the generation ...
Embodiment 2
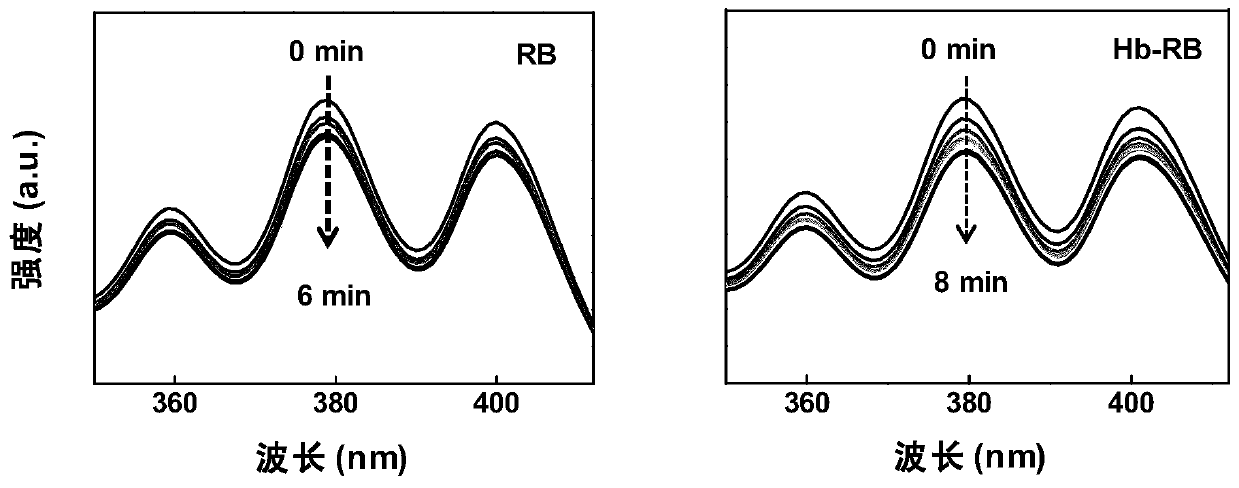
[0046] Prepare 0.01mg·mL under dark conditions -1 RB solution and the concentration of RB contained in it is 0.01mg·mL -1 Each 1mL of Hb-RB solution was mixed with 20μL of 1mg·mL -1 After the DMSO solution of DPA was mixed evenly, it was placed in a closed micro-quartz cuvette. Use a wavelength of 550nm and a power of 2mW / cm 2 Irradiate the cuvette with the exciting light, measure the absorbance of the system in the range of 350-410nm every minute, and compare the change of the absorbance at 380nm. Such as figure 2 As shown, it can be seen that with the prolongation of the irradiation time, the absorbance at 380 nm in the two systems decreases gradually, and the decrease is more obvious in the Hb-RB system. This phenomenon shows that under the same conditions, the Hb-RB system will generate more singlet oxygen.
Embodiment 3
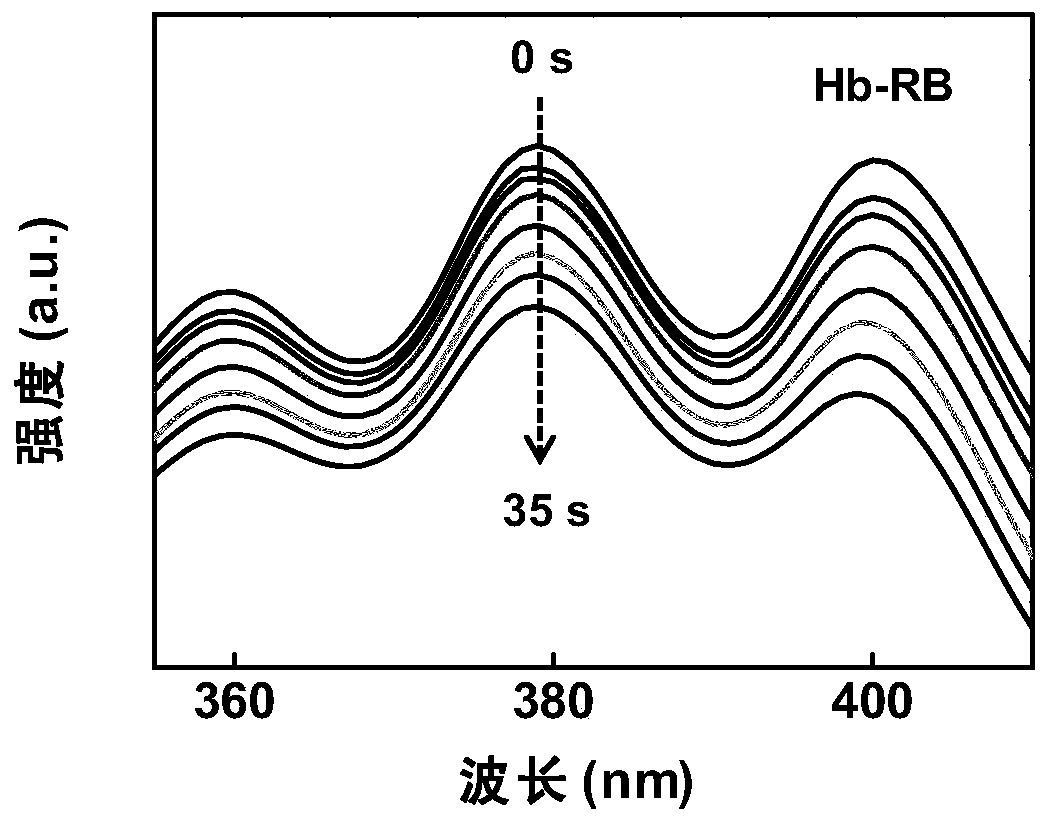
[0048] Prepare the RB concentration at 0.01mg·mL under dark conditions -1 3.5 mL of Hb-RB solution, with 70 μL of 1 mg·mL - 1 After the DMSO solution of DPA was mixed evenly, it was placed in a quartz cuvette. Nitrogen gas was first introduced to remove the molecular oxygen in the system, and then the system was exposed to indoor visible light conditions (the light intensity was 1.5mW / cm 2 ), feed oxygen, and measure the absorbance of the system in the range of 350-410nm every 5s. Such as image 3 As shown, it can be seen that the absorbance of the solution at 380nm has a significant decrease, that is, the system generates a large amount of singlet oxygen during the process of introducing oxygen, which further indicates that Hb-RB is very sensitive to excitation light, and there are molecules in the system Under the condition of oxygen, it can be excited by ordinary natural light, avoiding the use of high-energy lasers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com