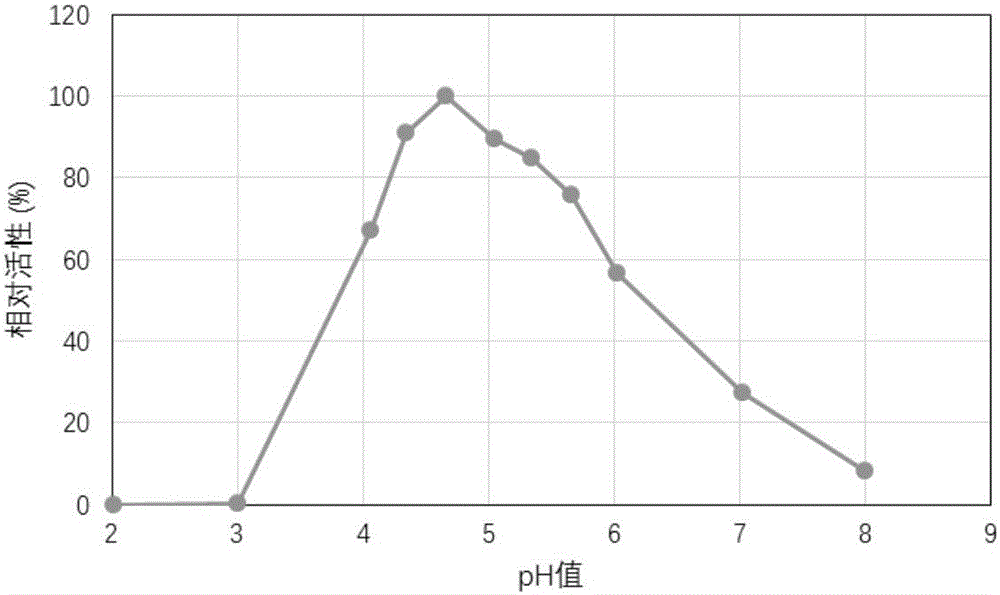
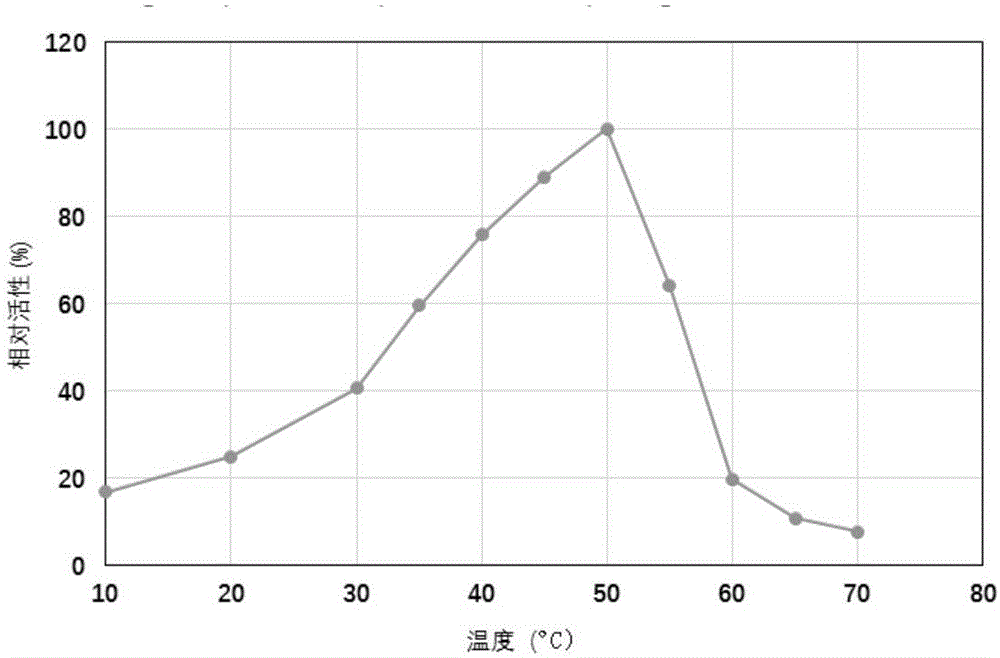
Protein with alpha-galactosidase activity and application of protein
A galactosidase, protein technology, applied in the application, glycosylase, enzyme and other directions, can solve the problems of affecting the digestion and absorption of nutrients, easy to produce flatulence and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1, the preparation of α-galactosidase galC
[0082] The present invention provides an alpha-galactosidase gene (galC gene) derived from Aspergillus oryzae, the sequence of the galC gene is sequence 2 in the sequence listing, and its coding sequence (CDS) is sequence 3 (sequence 3 The shown DNA fragment is named as the galC coding gene), the α-galactosidase (galC) shown in the 92-817th position of the sequence 3 coding sequence 1, the sequence 3 is carried out codon optimization, and the sequence encoding galC is obtained 43-2223, the galC-encoding gene shown in the 43-2223-position of Sequence 4 is recorded as the galC-Y encoding gene.
[0083] 1. Preparation of recombinant plasmids and recombinant bacteria
[0084] The DNA fragment between the EcoR I and Xba I recognition sequences of the pPICZαA plasmid was replaced with the galC coding gene shown in Sequence 3 to obtain a recombinant plasmid, which was named pPICZαA-galC. pPICZαA-galC can express the pro...
Embodiment 2
[0123] Example 2, α-galactosidase galC can degrade oligosaccharides in soybean milk
[0124] 1. Preparation of soymilk
[0125] Weigh 100g of soybeans, soak them in tap water overnight at room temperature, put them into a soymilk machine, and crush them to produce soymilk.
[0126] 2. galC can degrade oligosaccharides in soy milk
[0127] Three treatment groups and three control groups were set up in the experiment, and each group had two parallel experiments. The three treatments were 5 U / ml, 10 U / ml, and 15 U / ml of enzyme, that is, treatment 1, treatment 2 and treatment 3. The specific operation steps are as follows:
[0128] The operation steps of treatment 1, treatment 2 and treatment 3 are as follows: after adjusting the concentration of the fermentation supernatant in step 3 of Example 1, mix it with the soymilk supernatant in equal volumes to obtain a mixed solution, and the enzyme amount of galC in the mixed solution of treatment 1 is 5U / ml, the enzyme amount of ga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com