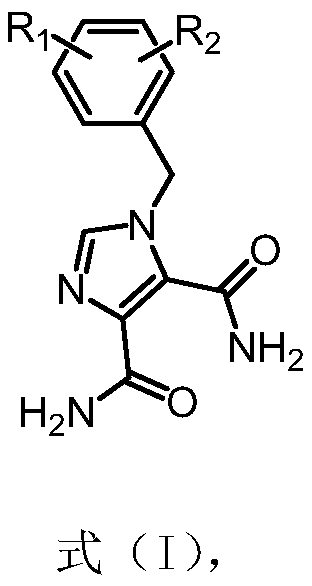
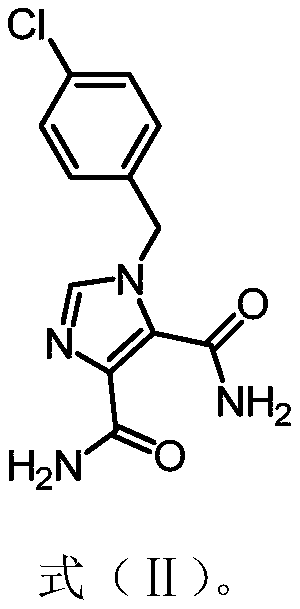
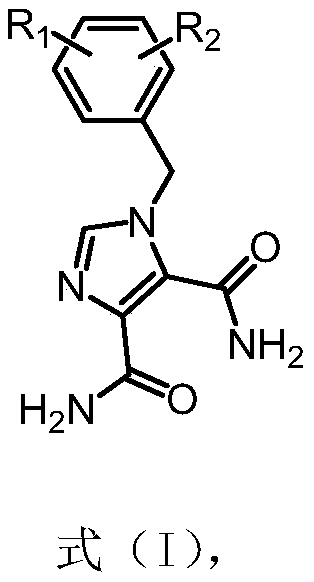
Application of n-benzyl imidamide derivatives as synergistic agents of polymyxin antibiotics
A technology of benzyl imidamide and polymyxin, which is applied in the field of biomedicine and can solve problems such as environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of 1-(2-chloro-6-fluorobenzyl)-1H-imidazole-4,5-diamide (15031)
[0053]
[0054] Step 1: Preparation of dimethyl 1-(2-chloro-6-fluorobenzyl)-1H-imidazole-4,5-dicarboxylate
[0055] Free dimethyl 1H-imidazole-4,5-dicarboxylate (9.2g, 50mmol, 1eq), 2-chloro-6-fluorobenzyl chloride (17.9g, 100mmol, 2eq) and potassium carbonate (17.3g, 125mmol, 2.5eq) were successively dissolved in 70mL N,N-dimethylformamide, stirred and reacted at 100°C for 15h, and monitored by TLC until the end point. The reaction solution was cooled to room temperature, 150 mL of water was added, extracted with ethyl acetate (100 mL×3), the ethyl acetate layers were combined, washed with water (100 mL×3) and saturated brine (100 mL×2), and dried over anhydrous sodium sulfate. Concentrate under reduced pressure to remove the organic solvent to obtain a colorless transparent liquid (dimethyl 1-(2-chloro-6-fluorobenzyl)-1H-imidazole-4,5-dicarboxylate), which is directly used in t...
Embodiment 2
[0059] Example 2 Preparation of 1-(4-chlorobenzyl)-1H-imidazole-4,5-diamide (15037)
[0060]
[0061] Step 1: Preparation of dimethyl 1-(4-chlorobenzyl)-1H-imidazole-4,5-dicarboxylate
[0062] Free dimethyl 1H-imidazole-4,5-dicarboxylate (7.6g, 41mmol, 1eq), p-chlorobenzyl chloride (13.2g, 82mmol, 2eq) and potassium carbonate (14.2g, 103mmol, 2.5eq) were sequentially dissolved In 50mL of N,N-dimethylformamide, the reaction was stirred at 90°C for 16h, and the reaction was monitored by TLC until the end point. The reaction solution was cooled to room temperature, 150 mL of water was added, extracted with ethyl acetate (100 mL×3), the ethyl acetate layers were combined, washed with water (100 mL×3) and saturated brine (100 mL×2), and dried over anhydrous sodium sulfate. Concentration under reduced pressure to remove the organic solvent gave the product as a colorless transparent liquid (1-(4-chlorobenzyl)-1H-imidazole-4,5-dicarboxylic acid dimethyl ester), which was directly...
Embodiment 3
[0066] Example 3 Preparation of 1-(3-chlorobenzyl)-1H-imidazole-4,5-diamide (15041)
[0067]
[0068] Step 1: Preparation of dimethyl 1-(3-chlorobenzyl)-1H-imidazole-4,5-dicarboxylate
[0069] Free dimethyl 1H-imidazole-4,5-dicarboxylate (5.0 g, 27 mmol, 1 eq), 3-chlorobenzyl bromide (11.5 g, 56 mmol, 2 eq) and potassium carbonate (9.3 g, 67.5 mmol, 2.5 eq) Dissolve in 50mL N,N-dimethylformamide successively, stir and react at 90°C for 10h, and monitor the reaction to the end by TLC. The reaction solution was cooled to room temperature, 150 mL of water was added, extracted with ethyl acetate (100 mL×3), the ethyl acetate layers were combined, washed with water (100 mL×3) and saturated brine (100 mL×2), and dried over anhydrous sodium sulfate. The organic solvent was removed by concentration under reduced pressure to obtain a colorless transparent liquid (1-(3-chlorobenzyl)-1H-imidazole-4,5-dicarboxylic acid dimethyl ester), which was directly used in the next reaction.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com