Preparation method of CO2-adsorbing shrimp-shell-base nitrogenous activated carbon
A technology of activated carbon and shrimp shells, applied in chemical instruments and methods, carbon compounds, inorganic chemistry, etc., can solve problems such as strong corrosion and complex process, and achieve the effect of low price, wide source, and excellent adsorption performance of recycling regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
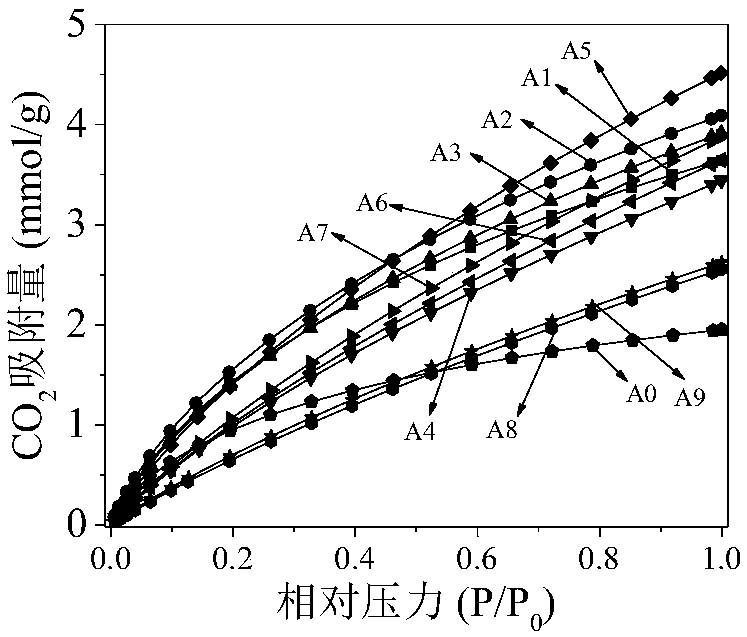
[0031] First, wash and dry the discarded shrimp shells at 110°C for 12 hours into powder to 100-140 mesh, then soak in excess 1mol / L hydrochloric acid for 12 hours, filter, wash with distilled water until neutral, and dry at 110°C for 12 hours to obtain shrimp Shell powder; then 2g shrimp shell powder, 2g potassium oxalate (the mass ratio of potassium oxalate and shrimp shell powder is 1:1) is mixed homogeneously in grinder, obtains mixture; Then mixture is placed in N 2 Carbonized material was obtained by carbonizing at 650°C for 2h (heating rate: 5°C / min) under the atmosphere (50ml / min); finally, the carbonized material was repeatedly washed with 1mol / L hydrochloric acid and distilled water until neutral, and dried at 110°C for 12h to obtain activated carbon. CO at normal temperature and pressure 2 The adsorption capacity is 3.64mmol / g (see figure 1 Middle curve A1), the nitrogen element content is 4.99wt%.
Embodiment 2
[0032] Example 2: Firstly, the discarded shrimp shells washed and dried at 110°C for 12 hours were powdered to 100-140 mesh, then soaked in excess 1mol / L hydrochloric acid for 12 hours, filtered, washed with distilled water until neutral, and dried at 110°C Obtain shrimp shell powder after 12h; Then mix 2g shrimp shell powder, 6g potassium oxalate (the mass ratio of potassium oxalate and shrimp shell powder is 3:1) in grinder, obtain mixture; Next mixture is placed in N 2 Carbonized material was obtained by carbonizing at 650°C for 2h (heating rate: 5°C / min) under the atmosphere (50ml / min); finally, the carbonized material was repeatedly washed with 1mol / L hydrochloric acid and distilled water until neutral, and dried at 110°C for 12h to obtain activated carbon. CO at normal temperature and pressure 2 The adsorption capacity is 4.09mmol / g (see figure 1 In curve A2), the nitrogen element content is 6.38wt%.
Embodiment 3
[0033] Example 3: Firstly, the discarded shrimp shells washed and dried at 110°C for 12 hours were powdered to 100-140 mesh, then soaked in excess 1mol / L hydrochloric acid for 12 hours, filtered, washed with distilled water until neutral, and dried at 110°C Obtain shrimp shell powder after 12h; Then 2g shrimp shell powder, 10g potassium oxalate (the mass ratio of potassium oxalate and shrimp shell powder is 5:1) are mixed in grinder, obtain mixture; Next mixture is placed in N 2Carbonized material was obtained by carbonizing at 650°C for 2h (heating rate: 5°C / min) under the atmosphere (50ml / min); finally, the carbonized material was repeatedly washed with 1mol / L hydrochloric acid and distilled water until neutral, and dried at 110°C for 12h to obtain activated carbon. CO at room temperature and pressure 2 The adsorption capacity is 3.91mmol / g (see figure 1 Middle curve A3), the nitrogen element content is 6.45wt%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com