Sorafenib alpha-amino butyrate and preparation method thereof
A technology of aminobutyrate and sorafenib, applied in the directions of organic chemistry, organic chemistry, antitumor drugs, etc., can solve the problems of unfavorable industrial method production, large molecular weight of toluenesulfonic acid, unfavorable long-term administration, etc. Facilitate industrial production, improve medicinal safety, and have good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
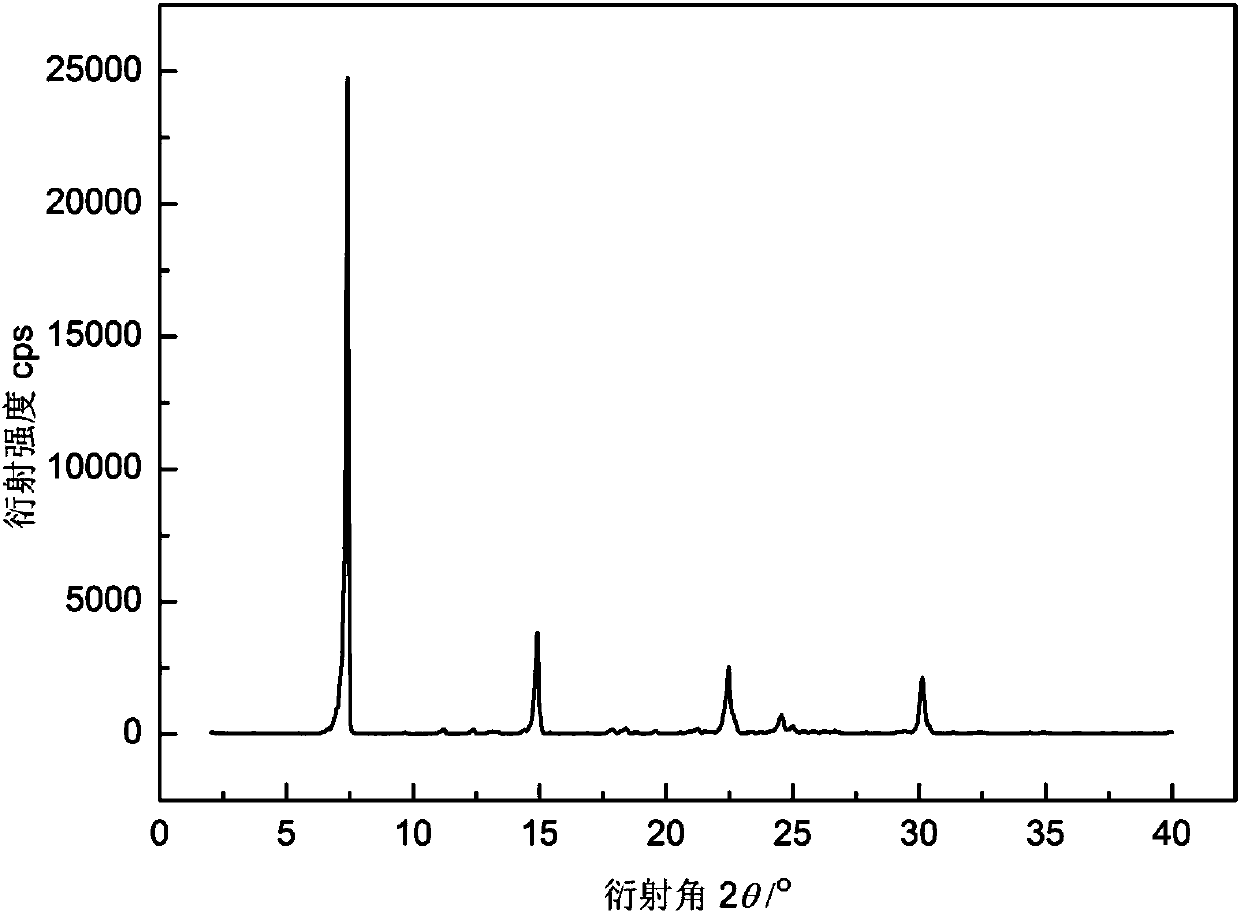
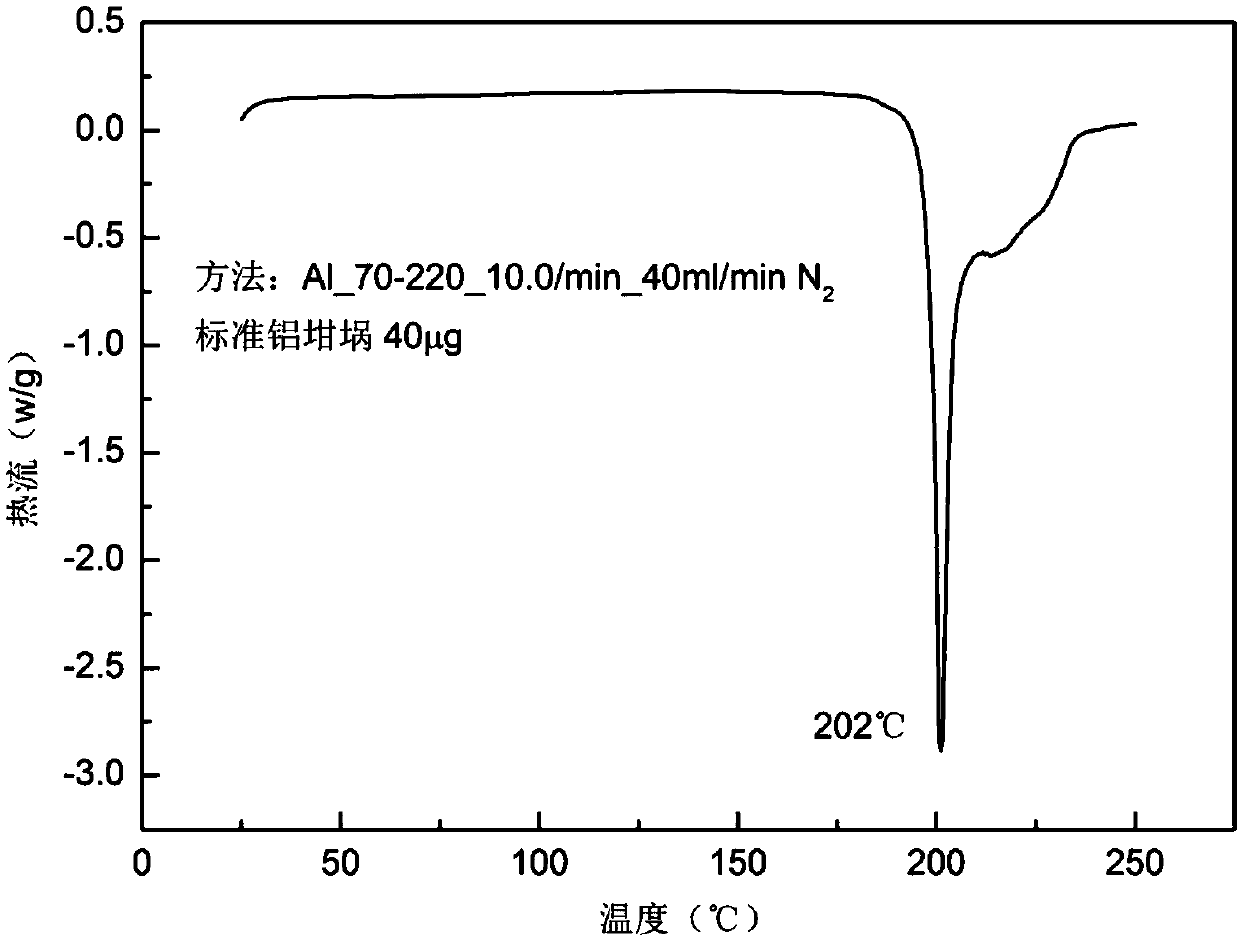
[0038] Mix 200g of crystal form sorafenib, 70g of α-aminobutyric acid, 2L of ethanol, and 1L of water in a reaction flask, heat to 80°C, and carry out reaction crystallization for 0.5 hours; then cool to 10°C at a rate of 3°C / min, The obtained product was filtered and fully dried to obtain 235g of Sorafenib α-aminobutyrate, with a yield of 96.3% and a purity of 99.9%. The powder X-ray diffraction pattern of the product and figure 1 In agreement, the DSC spectrum is consistent with figure 2 Consistent, the product microscope photos are attached image 3 , the main particle size is 15μm.
Embodiment 2
[0040] Add 200g of amorphous sorafenib, 44g of α-aminobutyric acid, 1.5L of n-butanol, and 0.5L of water into the reaction flask, heat to 75°C, and fully react for 3 hours. Cool to 20 DEG C with the rate of 1 DEG C / min then, the product obtained is filtered, washed, obtains 237g Sorafenib α-aminobutyrate after fully drying, yield 97.0%, purity 99.9%. The powder X-ray diffraction pattern of the product has characteristic peaks at 3.7, 5.5, 7.4, 11.2, 12.4, 14.4, 14.9, 18.4, 21.3, 22.5, 24.5, 25.0, 30.1 degrees, and the DSC spectrum has an endothermic peak at 202 ° C.
Embodiment 3
[0042] Add 200g of sorafenib in crystal form, 89g of α-aminobutyric acid, 2L of ethanol, 2.7L of n-propanol, and 230mL of water into the reaction flask, heat to 70°C, and fully react for 1.5 hours. Cool to 15 DEG C with the rate of 1.5 DEG C / min then, the product obtained is filtered, washed, obtains 233g Sorafenib α-aminobutyrate after fully drying, yield 95.3%, purity 99.9%. The powder X-ray diffraction pattern of the product has characteristic peaks at 3.7, 5.5, 7.4, 11.2, 12.4, 14.4, 14.9, 18.4, 21.3, 22.5, 24.5, 25.0, 30.1 degrees, and the DSC spectrum has an endothermic peak at 202 ° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com