Axially-chiral aggregation-induced luminous compound as well as preparation method and application thereof
A compound and axial chirality technology, applied in the field of axial chiral compounds and their preparation, can solve the problems of product solubility easily affected by photo-oxidation, long synthetic route steps, large synthetic workload, etc., and achieves correct structure, good stability, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The reaction formula is as follows:
[0027]
[0028] Add 1.0g (2.9mmol) 2,2'-dihydroxy-[1,1'-binaphthyl]-3,3'-dialdehyde racemate, 50mL ethanol, 0.94g (6.0mmol) diethyl malonate and 0.25g (2.9mmol) piperidine were heated to reflux for 24h, cooled to room temperature, a solid precipitated, filtered, and dried to obtain 1.1g of a yellow powder product with a yield of 70%.
[0029] In the above examples, the racemate of 2,2'-dihydroxy-[1,1'-binaphthyl]-3,3'-dialdehyde was replaced by S-type 2,2'-dihydroxy-[1, 1'-binaphthyl]-3,3'-dialdehyde, and the other steps are exactly the same as those in the above examples to obtain the S-type product.
[0030] In the above examples, the racemate of 2,2'-dihydroxy-[1,1'-binaphthyl]-3,3'-dialdehyde was replaced by R-type 2,2'-dihydroxy-[1, 1'-binaphthyl]-3,3'-dialdehyde, and the other steps are exactly the same as in the above examples to obtain the R-type product.
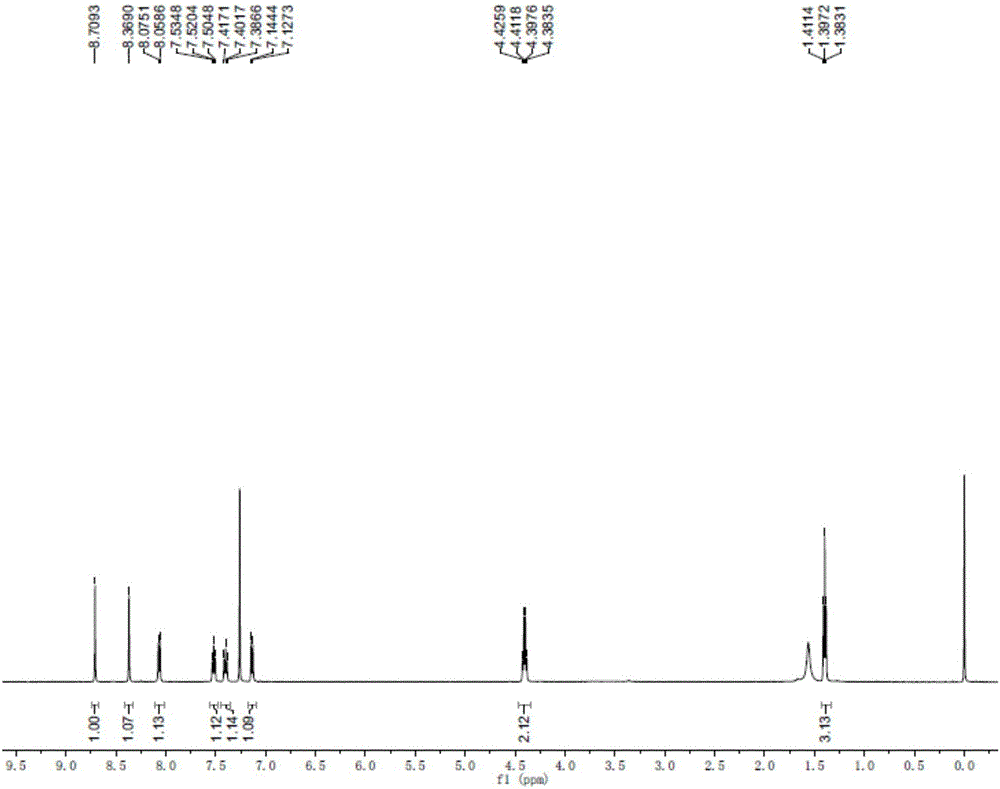
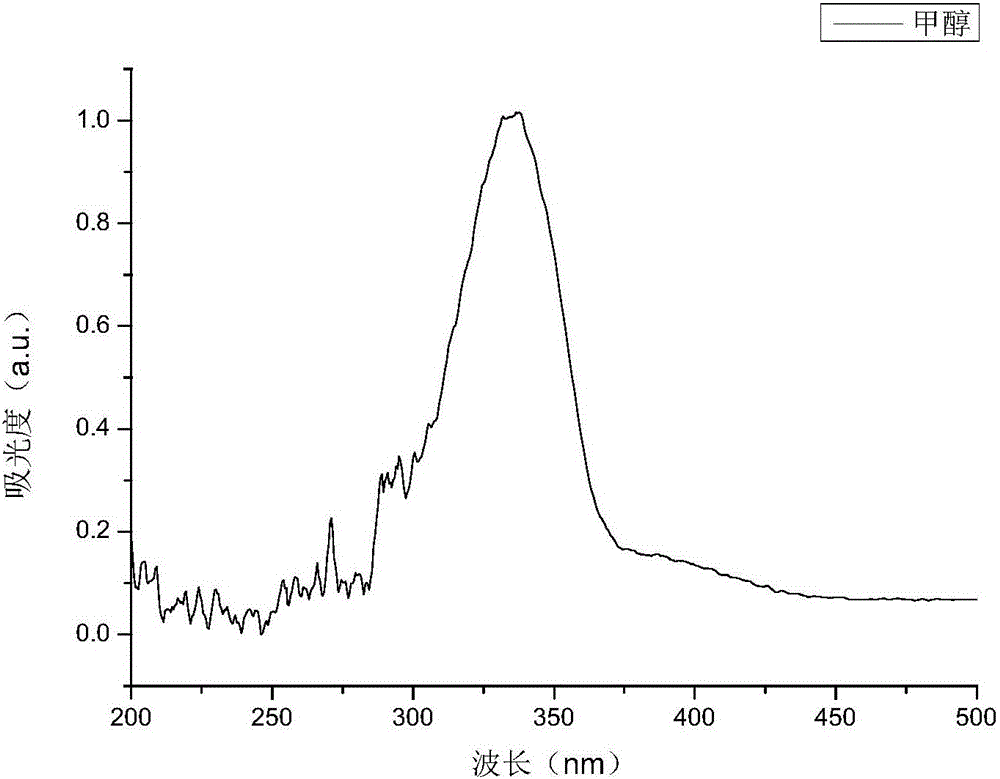
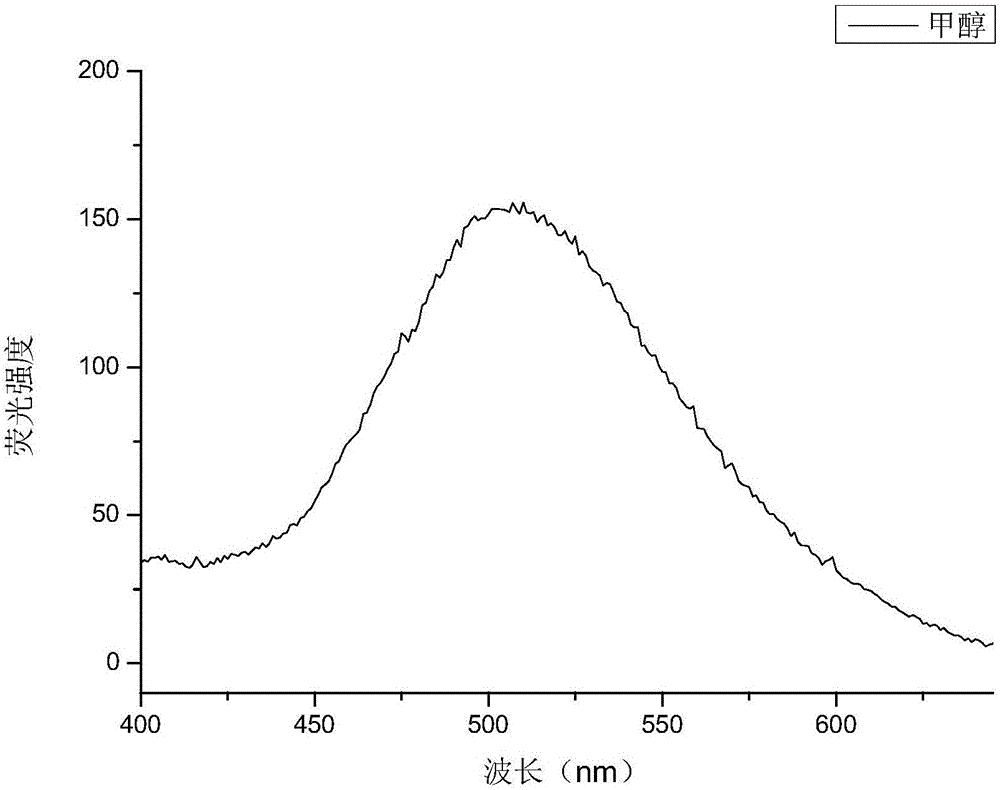
[0031] The structure detection result of this compound is as f...
Embodiment 2
[0037] The reaction formula is as follows:
[0038]
[0039]Add 1.0g (2.9mmol) 2,2'-dihydroxy-[1,1'-binaphthyl]-3,3'-dialdehyde racemate, 50mL ethanol, 0.78g (6.0mmol) ethyl acetoacetate and 0.25g (2.9mmol) piperidine were heated to reflux for 24h, cooled to room temperature, a solid precipitated, filtered and dried to obtain 1.0g of a yellow powder product with a yield of 73%.
[0040] In the above examples, the racemate of 2,2'-dihydroxy-[1,1'-binaphthyl]-3,3'-dialdehyde was replaced by S-type 2,2'-dihydroxy-[1, 1'-binaphthyl]-3,3'-dialdehyde, and the other steps are exactly the same as those in the above examples to obtain the S-type product.
[0041] In the above examples, the racemate of 2,2'-dihydroxy-[1,1'-binaphthyl]-3,3'-dialdehyde was replaced by R-type 2,2'-dihydroxy-[1, 1'-binaphthyl]-3,3'-dialdehyde, and the other steps are exactly the same as in the above examples to obtain the R-type product.
[0042] The structure detection result of this compound is as f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com