Application of 1,8-dihexanoyl emodin in preparation of anti-HIV-1 drugs
A technology of dihexanoyl-emodin and emodin, which is applied in the application field of 1,8-dihexanoyl-emodin in the preparation of anti-HIV-1 drugs, and can solve the problems of easy oxidative deterioration, poor stability, and poor solubility of emodin, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] [Example 1] Preparation, purification and identification of emodin derivatives
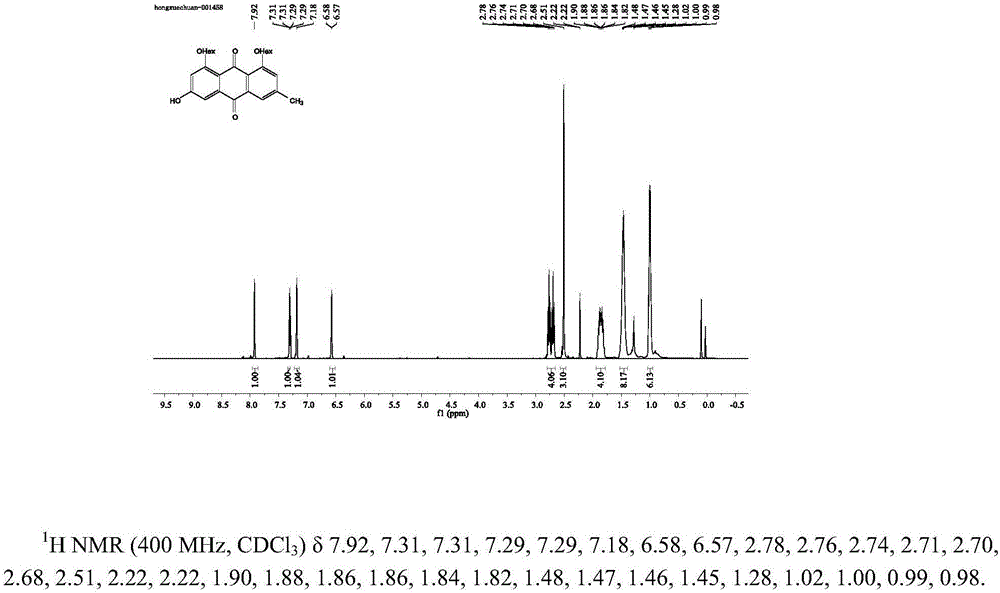
[0041] 3-emodin acetate: take 125mg emodin and 138mg potassium carbonate, add 5mL acetone, and react under reflux at 50°C. After the reaction, add hydrochloric acid to adjust the pH value to 1-2, filter to obtain a red solid, and dry it in a vacuum oven. Add 74.3 mg of the product to a round bottom flask, add 34.7 mg of sodium hydroxide and 15 mL of ethanol, stir at 30 ° C for 4 h, put the reaction solution until no liquid precipitates, dilute with water, adjust the pH value to 1-2 with hydrochloric acid, and extract with ethyl acetate The aqueous phase was combined with the organic phases, dried over sodium sulfate and dried to obtain a red solid, and the nuclear magnetic identification product was 3-acetate emodin ( figure 1 ).
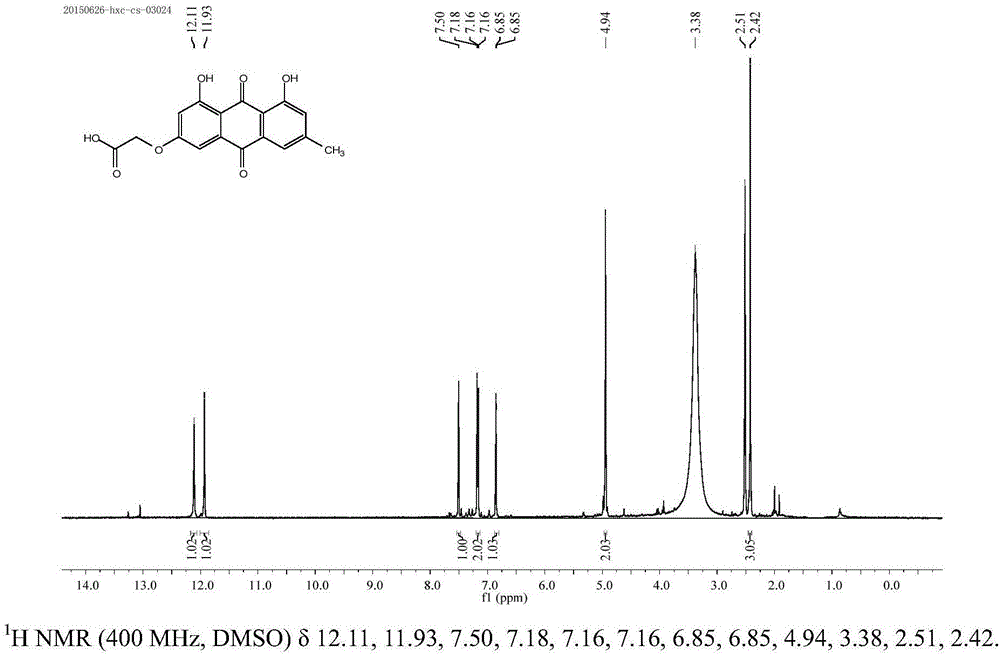
[0042] 1,8-Dihexanoyl-emodin: Dissolve 1g of emodin in a 50mL round-bottomed flask, operate in anhydrous and oxygen-free manner, add 20mL of anhydrous pyridine...
Embodiment 2
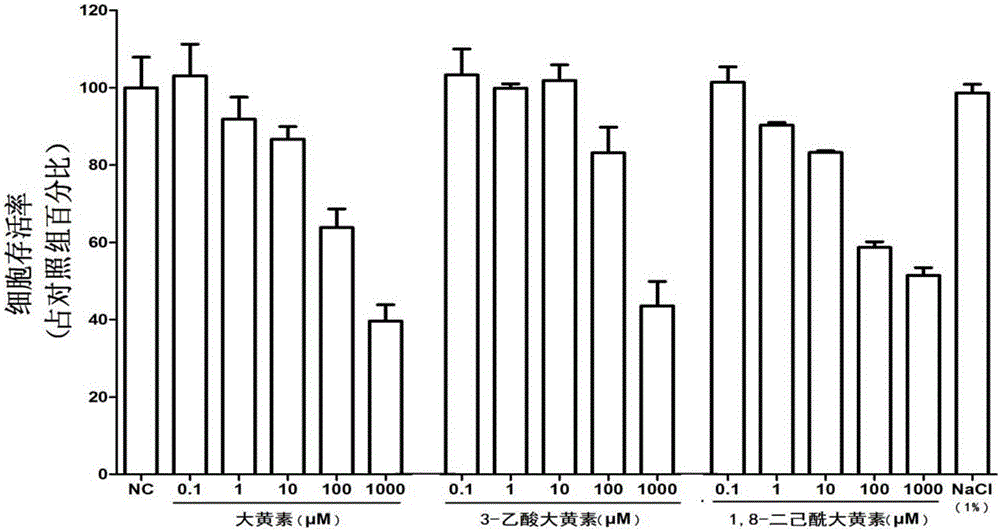
[0043] [Example 2] MTT method to detect the cytotoxic effect of emodin derivatives 3-emodin acetate and 1,8-dihexanoyl emodin
[0044]Anticoagulated blood from healthy blood donors was mixed with lymphatic separation fluid (Organon Teknika Corp, Durham), and centrifuged at 1500 g for 45 min to separate mononuclear cells. The mononuclear cell layer was collected, suspended with DMEM, and inoculated into 2% gelatin-coated culture dishes, incubated at 37°C for 45 min, and then washed with DMEM to remove unadhered cells. After the adherent cells were digested with EDTA, they were resuspended with complete DMEM (10% FCS, 2 mmol / mL glutamine, 100 U / mL penicillin, 100 μg / mL streptomycin, and non-essential amino acids), and mixed with 10 5 per well in a 96-well plate. After preliminary purification, non-specific esterase staining and fluorescent screening of CD14 monoclonal antibody (Leu-M3) and low-density lipoprotein (LDL) confirmed that 98.5% of the cells in the well were monocyte...
Embodiment 3
[0048] [Example 3] Anti-HIV-1 effect of emodin derivatives 3-acetate emodin and 1,8-dihexanoyl emodin in vitro
[0049] After HIV-1Bal strain infects human macrophages for 2 hours (using RT-PCR and ELISA to determine the establishment of HIV-1 infection), discard the virus liquid, and add 10 μM emodin, 3-acetate emodin or 1 , 8-dihexanoyl-emodin in complete DMEM culture solution, cultivated at 37°C, and collected cells and cell supernatant on the 8th day after infection. The expression of Gag mRNA in the cells was detected by RT-PCR; the content of p24 in the supernatant of the cells was detected by ELISA, and the expression of p24 in the cells was measured by Western blotting, and 3-acetate emodin and 1,8-dihexanoyl emodin were determined by the above three aspects Antiviral effects against HIV-1. Specific steps are as follows:
[0050] (1) Real-time quantitative RT-PCR was used to detect the expression of Gag gene, GAPDH was used as an internal reference, and the control g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com