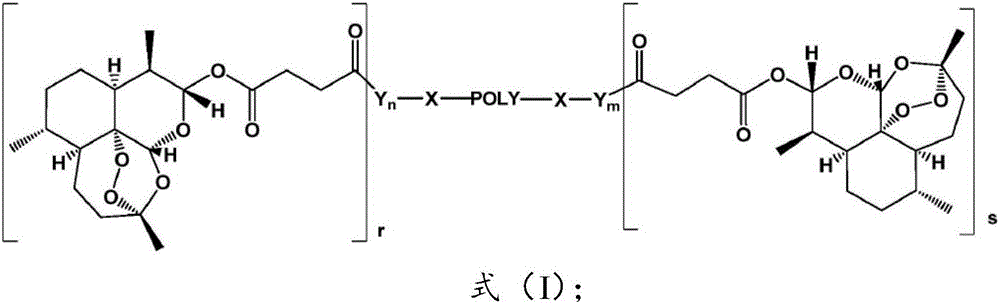
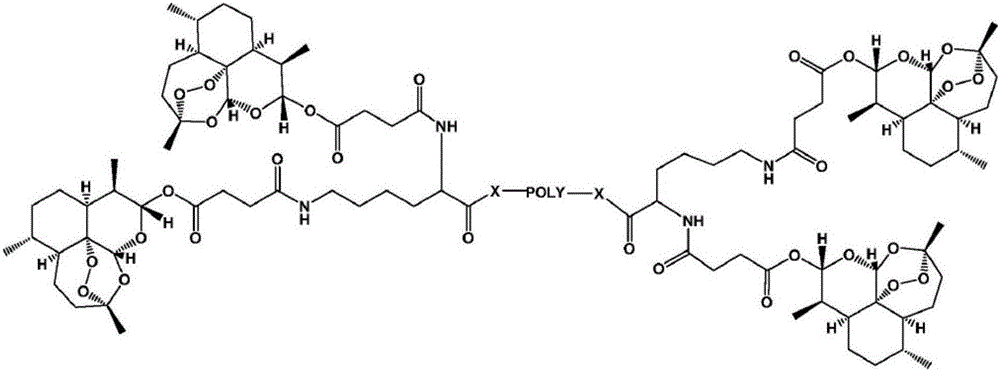
Application of polyethylene glycol artesunate in preparation of antitumor drug
A technology of polyethylene glycol Artemisia annua and anti-tumor drugs, which is applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problem of unsatisfactory tumor inhibition effects, and achieve the effect of inhibiting proliferation and having a significant dose-effect relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Inhibitory effect of polyethylene glycol artesunate on proliferation of different tumor cell lines
[0035] 1. Test material
[0036] Polyethylene glycol artesunate Ⅰ, Ⅱ, Ⅲ, Ⅳ, made by Kunming Pharmaceutical Research Institute according to the methods disclosed in Chinese patent CN103450468B Example 1, Example 3, Example 2 and Implementation 4; artesunate, Chongqing Huafang Wulingshan Pharmaceutical Co., Ltd.; doxorubicin hydrochloride for injection (ADR), Zhejiang Hisun Pharmaceutical Co., Ltd.; human liver cancer cell line (HepG-2), human colon cancer cell line (HT-29), Human lung adenocarcinoma cell line (SPC-A-1), human prostate cancer cell line (PC-3), human gastric cancer cell line (MKN-28), human laryngeal cancer cell line (Hep2), human cervical cancer cell line ( Hela), human non-small cell lung cancer cell line (A549), human breast cancer cell line (MCF-7), human chronic myeloid leukemia cell line (K562), human promyelocytic leukemia cell line (HL-60...
Embodiment 2
[0056] Example 2 In vitro toxicity test of polyethylene glycol artesunate to normal cell human embryonic liver cells (L-02)
[0057] 1. Test material
[0058] Polyethylene glycol artesunate Ⅰ, Ⅱ, Ⅲ, Ⅳ, made by Kunming Pharmaceutical Research Institute according to the methods disclosed in Chinese patent CN103450468B Example 1, Example 3, Example 2 and Implementation 4; artesunate, Chongqing Huafang Wulingshan Pharmaceutical Co., Ltd.; human embryonic liver cell L-02, cell bank of Shanghai Institute of Biological Sciences, Chinese Academy of Sciences; XB-K-25 cell counting plate, glass instrument factory, Haimen County, Jiangsu Province; ZSBB-724 constant temperature water bath Pot, Shanghai Medical Constant Temperature Equipment Factory; synergy 2 multifunctional microplate reader, BioTek Company; HERA cell 150 C02 incubator, Thermo; RJ-TDL-508 cell centrifuge, Wuxi Ruijiang Centrifuge Factory; 96-well plate, Corning Corning.
[0059] 2. Test method
[0060] 2.1 Preparation ...
Embodiment 3
[0074] Example 3 Effect of polyethylene glycol artesunate on the growth of mouse transplanted sarcoma S180
[0075] 1. Experimental materials
[0076] Polyethylene glycol artesunate Ⅰ, Ⅱ, Ⅲ, Ⅳ, made by Kunming Pharmaceutical Research Institute according to the methods disclosed in Chinese patent CN103450468B Example 1, Example 3, Example 2 and Implementation 4; artesunate, Chongqing Huafang Wulingshan Pharmaceutical Co., Ltd.; S180 cell line, purchased from the cell bank of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences; Kunming mice, 18-22g, half female, provided by the Experimental Animal Center of Kunming Pharmaceutical Group.
[0077] 2. Experimental method
[0078] 2.1 Drug preparation: Polyethylene glycol artesunate Ⅰ, Ⅱ, Ⅲ, Ⅳ and artesunate were prepared with normal saline to make three concentrations of 0.5, 1.0, 1.5mg / ml low, medium and high respectively, according to 0.2ml / 10g administration, the administration dosage is 10, 20, 30mg / kg r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com