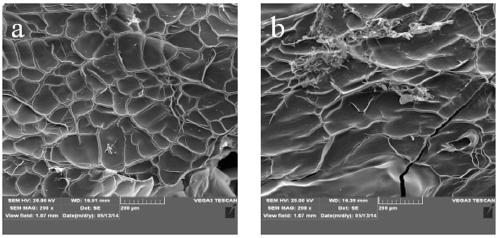
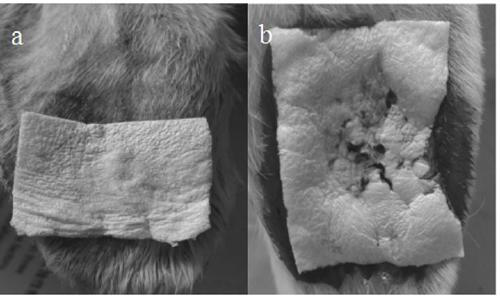
Method for preparing internal hemostatic dressing by programmed cooling
A technology of hemostatic dressing and cooling rate, which is applied in the field of preparation of hemostatic dressings in vivo, can solve the problems of affecting the hemostatic material's absorption of oozing blood on the wound surface, long clinical hemostasis time of sponge, uneven surface of hemostatic material, etc. Hidden danger, difficult secondary bleeding, and the effect of improving the hemostatic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] A preparation method of an internal hemostatic dressing, comprising the following steps:
[0028] Step 1: Dissolve humanoid collagen in water for injection, stir to dissolve it completely, so that the mass concentration of humanoid collagen in the solution is 1%-5%. A human-derived collagen produced by high-density fermentation;
[0029] Step 2: Transfer the solution into a disposable sterile Petri dish or mold, 7-15 mL per dish or mold;
[0030] Step 3: Use the program freezer to control the cooling rate at 4-8°C / min during the pre-freezing process for precise cooling down to -40--80°C;
[0031] Step 4: Freeze-dry the sample in a vacuum freeze dryer for 24-48 h;
[0032] Step 5: Place the freeze-dried sample in a vacuum drying oven for cross-linking, adjust the cross-linking temperature to 100°C-150°C, and the cross-linking time to 24h-96h;
[0033] Step 6: Sterilize by irradiation for 10-30 hours.
Embodiment 1
[0035] Dissolve the human-like collagen in water for injection, stir to dissolve it completely, so that the mass concentration of the human-like collagen in the mixed solution is 1%, and then place it in a programmed freezer to freeze at a rate of -4°C / min to -80°C and kept for 2 h, quickly transferred to a vacuum freeze dryer to freeze-dry for 24 h, then transferred to a vacuum oven at 100°C for cross-linking for 24 h, took out the package and irradiated with Co60 and sterilized for 24 h to obtain Internal hemostatic dressing.
Embodiment 2
[0037] Dissolve human-like collagen in water for injection, stir to dissolve completely, so that the mass concentration of human-like collagen in the mixed solution is 2%, and then place it in a programmed freezer at a rate of -4°C / min to - 80°C and kept for 2 h, quickly transferred to a vacuum freeze dryer to freeze-dry for 40 h, then transferred to a vacuum drying oven at 110°C for cross-linking for 28 h, took out the package for Co60 irradiation cross-linking and sterilized for 24 h , to obtain internal hemostatic dressings.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com