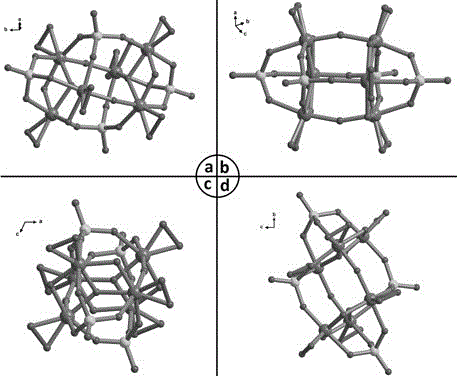
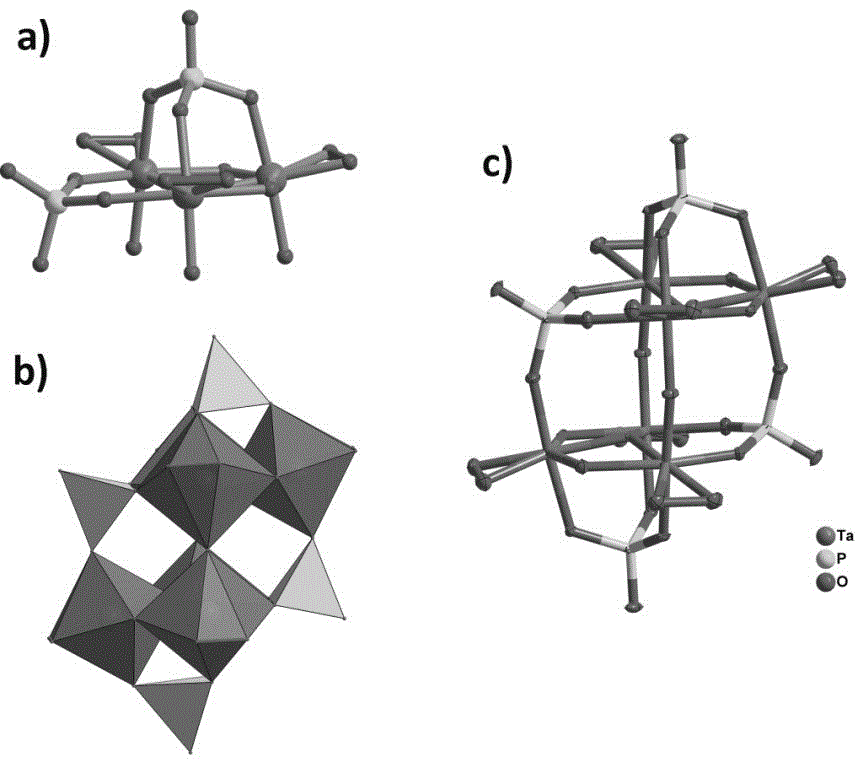Novel heterotantalate and preparation method and application thereof
A technology of tantalate and heteropoly, which is applied in the field of new heteropoly tantalate and its preparation, can solve the problems of little research and no reports of heteropoly tantalate, and achieve a clear structure, low cost and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A preparation method of a novel heteropolytantalate, comprising the following steps:
[0024] (1) Synthesize potassium hexatantalate K according to conventional methods in this field 8 [Ta 6 o 19 ]·16H 2 O precursor (specific references Nelson W H, Tobias R S, Inorg. Chem., 1963, 2, 985–992);
[0025] (2) Under stirring conditions, mix 1.50 g K 8 [Ta 6 o 19 ]·16H 2 O (0.75 mmol) was dissolved in hydrogen peroxide solution (obtained by mixing 13.5 ml of 30 wt% hydrogen peroxide and 165 ml of water), added 6.5 ml of 3 mol / L phosphoric acid solution, stirred at room temperature for 15 minutes, and adjusted the pH to 2.5, reacted at 90°C for 3 hours, cooled to room temperature, added 0.56 g of potassium chloride (7.5 mmol) and stirred at room temperature for 30 min, filtered to remove the precipitate and stood for 8 days to obtain crystals, which were post-treated (washed, dried ) That is, the yield is 71%.
Embodiment 2
[0027] A preparation method of a novel heteropolytantalate, comprising the following steps:
[0028] (1) Synthesize potassium hexatantalate K according to conventional methods in this field 8 [Ta 6 o 19 ]·16H 2 O precursor (specific references Nelson W H, Tobias R S, Inorg. Chem., 1963, 2, 985–992).
[0029] (2) Under stirring conditions, mix 1.50 g K 8 [Ta 6 o 19 ]·16H 2 O (0.75 mmol) was dissolved in hydrogen peroxide solution (consisting of 13.5 ml of 30 wt% hydrogen peroxide and 165 ml of water), and 6.2 ml of 3 mol / L phosphoric acid solution was added, stirred at room temperature for 15 minutes, and then adjusted to pH 2.3 with sodium hydroxide solution. React at 85 °C for 4 hours, cool to room temperature, add 0.56 g of potassium chloride (7.5 mmol) and stir at room temperature for 30 min, filter to remove the precipitate and place it for 10 days to obtain crystals, which are post-treated (washed and dried) Obtained, the yield is 53%.
Embodiment 3
[0031] A preparation method of a novel heteropolytantalate, comprising the following steps:
[0032] (1) Synthesize potassium hexatantalate K according to conventional methods in this field 8 [Ta 6 o 19 ]·16H 2 O precursor (specific references Nelson W H, Tobias R S, Inorg. Chem., 1963, 2, 985–992);
[0033] (2) Under stirring conditions, mix 1.50 g K 8 [Ta 6 o 19 ]·16H 2 O (0.75 mmol) was dissolved in hydrogen peroxide solution (consisting of 13.5 ml 30 wt% hydrogen peroxide and 165 ml water), added 6.5 ml 3 mol / L phosphoric acid solution, stirred at room temperature for 15 minutes, and adjusted the pH to 2.1 with sodium hydroxide solution. React at 80 °C for 3 hours, cool to room temperature, add 0.56 g of potassium chloride (7.5 mmol) and stir at room temperature for 30 min, filter to remove the precipitate and place it for 10 days to obtain crystals, which are post-treated (washed and dried) The yield was 31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com