Chemical method of preparing fructose from glucose
A glucose and fructose technology, applied in chemical instruments and methods, preparation of sugar derivatives, organic chemistry, etc., can solve the problems of low fructose yield and selectivity, low glucose concentration, harsh reaction conditions, etc., and achieve simple and easy reaction operation The effect of high concentration and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
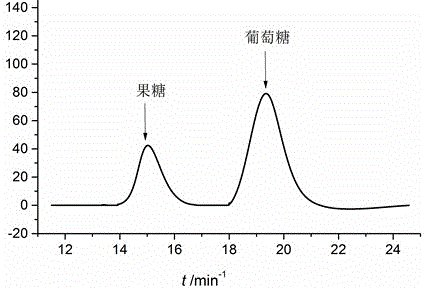
Image
Examples
Embodiment 1
[0016] Example 1 Add raw material glucose and catalyst p-hydroxybenzoic acid to the aqueous solution so that their mass fractions are 0.36% and 0.27% respectively. The solution was reacted at 90° C. for 10 h at an atmospheric pressure, and cooled to room temperature after the reaction. The following results were obtained: the conversion rate of glucose was 49.5%, the yield of fructose was 48.1%, and the selectivity of fructose was as high as 97.0%.
[0017] Example catalyst Glucose conversion rate% Fructose yield % selectivity% Example 2 3-Chloro-4-hydroxybenzoic acid 35.7 33.9 96.1 Example 3 p-Chlorophenol 45.3 43.4 95.8 Example 4 2,4-Dichlorophenol 34.4 33.1 96.2 Example 5 Salicylamide 48.1 40.1 83.4 Example 6 4-Hydroxysalicylamide 44.9 34.4 76.6
[0018] Note Table 1: Examples 2-6 are the comparison results with Example 1. Except for the different types of catalysts, other parameters are the same as Exa...
Embodiment 7
[0019] Example 7 Add raw material glucose and catalyst p-hydroxybenzoic acid to the aqueous solution so that the mass fractions are 0.36% and 0.027% respectively. The solution was reacted at 90° C. for 10 h at an atmospheric pressure, and cooled to room temperature after the reaction. The following results were obtained: the conversion rate of glucose was 41.7%, the yield of fructose was 40.5%, and the selectivity was as high as 97.1%.
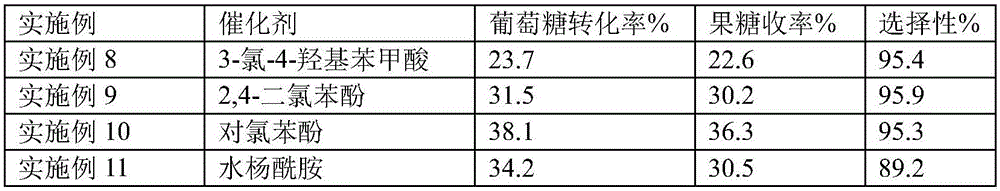
[0020] Table 2 is the experimental result of comparative example 7
[0021]
[0022]
[0023] Note Table 2: Examples 8-12 are the comparison results with Example 7, except that the type of catalyst is different, other parameters are the same as Example 7.
Embodiment 13
[0024] Example 13 The raw material glucose and the catalyst p-hydroxybenzoic acid were added into the aqueous solution so that their mass fractions were 50% and 0.75% respectively. The solution was reacted at 90° C. under one atmospheric pressure for 2 h and then cooled to room temperature. The following results were obtained: the conversion rate of glucose was 35.9%, the yield of fructose was 34.8%, and the selectivity was 96.9%.
[0025] Example catalyst Glucose conversion rate% Fructose yield % selectivity% Example 14 3-Chloro-4-hydroxybenzoic acid 23.8 22.6 95.0 Example 15 p-Chlorophenol 36.5 34.9 95.6
[0026] Note Table 3: Examples 14-15 are the comparison results with Example 13, except that the type of catalyst is different, other parameters are the same as Example 13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com